ARF function does not require p53 stabilization or Mdm2 relocalization
- PMID: 11739734
- PMCID: PMC134207
- DOI: 10.1128/MCB.22.1.196-206.2002
ARF function does not require p53 stabilization or Mdm2 relocalization
Abstract
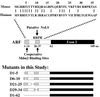
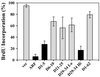
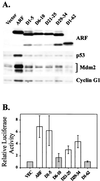
It is generally accepted that the ARF tumor suppressor induces p53-dependent growth arrest by sequestering the p53 antagonist Mdm2 in the nucleolus. Previous mutagenic studies of murine ARF suggested that residues 1 through 14 and 26 through 37 were critical for Mdm2 binding, while the latter domain also governed ARF nucleolar localization. We show that mouse ARF residues 6 to 10 and 21 to 25 are required for ARF-induced growth arrest whereas residues 1 to 5 and 29 to 34 are dispensable. Deletion of the putative nucleolar localization signal (31)RRPR(34) did not prevent nucleolar localization. Surprisingly, unlike wild-type ARF, growth-inhibitory mutants D1-5 and D29-34 failed to stabilize p53 yet induced its transcriptional activation in reporter assays. This suggests that p53 stabilization is not essential for ARF-mediated activation of p53. Like wild-type ARF, both mutants also exhibited p53-independent function since they were able to arrest p53/Mdm2-null cells. Notably, other mutants lacking conserved residues 6 to 10 or 21 to 25 were unable to suppress growth in p53-positive cells despite nucleolar localization and the ability to import Mdm2. Those observations stood in apparent contrast to the ability of wild-type ARF to block growth in some cells without relocalizing endogenous Mdm2 to nucleoli. Together, these data show a lack of correlation between ARF activity and Mdm2 relocalization, suggesting that additional events other than Mdm2 import are required for ARF function.
Figures
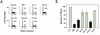





References
-
- Agarwal, M. L., W. R. Taylor, M. V. Chernov, O. B. Chernova, and G. R. Stark. 1998. The p53 network. J. Biol. Chem. 273:1–4. - PubMed
-
- Chin, L., J. Pomerantz, and R. A. DePinho. 1998. The INK4a/ARF tumor suppressor: one gene—two products—two pathways. Trends Biochem. Sci. 23:291–296. - PubMed
-
- Cong, F., X. Zou, K. Hinrichs, K. Calame, and S. P. Goff. 1999. Inhibition of v-Abl transformation by p53 and p19ARF. Oncogene 18:7731–7739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
