The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells
- PMID: 11739746
- PMCID: PMC134226
- DOI: 10.1128/MCB.22.1.343-356.2002
The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells
Abstract
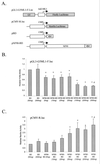
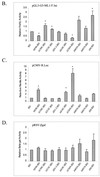
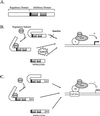
Nuclear factor 90 (NF90) was originally isolated in a complex that binds to the antigen recognition response element (ARRE-2) present in the interleukin-2 promoter. To characterize the transcriptional properties of NF90 in mammalian cells, we examined its ability to modulate promoter function in cellular transfection assays. NF90-Gal4 fusion proteins inhibited transcription from the adenovirus major late promoter in a fashion that was dependent on Gal4 targeting. Conversely, NF90 activated the cytomegalovirus immediate-early promoter, to which it was not targeted. These effects required distinct but overlapping domains in the C terminus of NF90, which contains a functional nuclear localization signal and two double-stranded-RNA binding motifs. NF90 is present in cellular complexes together with the NF45 protein. Transfection assays showed that NF45 binds NF90 strongly and stimulates its ability to activate but not to inhibit gene expression. This report characterizes NF90 as both a positive and negative regulator of gene expression, depending on the promoter context, and suggests a role for NF45 as a regulator of NF90.
Figures






Similar articles
-
Selective regulation of gene expression by nuclear factor 110, a member of the NF90 family of double-stranded RNA-binding proteins.J Mol Biol. 2003 Sep 5;332(1):85-98. doi: 10.1016/s0022-2836(03)00885-4. J Mol Biol. 2003. PMID: 12946349
-
Nuclear factor 90 is a substrate and regulator of the eukaryotic initiation factor 2 kinase double-stranded RNA-activated protein kinase.J Biol Chem. 2001 Aug 31;276(35):32522-30. doi: 10.1074/jbc.M104408200. Epub 2001 Jul 3. J Biol Chem. 2001. PMID: 11438540
-
The RNA binding complexes NF45-NF90 and NF45-NF110 associate dynamically with the c-fos gene and function as transcriptional coactivators.J Biol Chem. 2015 Oct 30;290(44):26832-45. doi: 10.1074/jbc.M115.688317. Epub 2015 Sep 17. J Biol Chem. 2015. PMID: 26381409 Free PMC article.
-
NF90 in posttranscriptional gene regulation and microRNA biogenesis.Int J Mol Sci. 2013 Aug 19;14(8):17111-21. doi: 10.3390/ijms140817111. Int J Mol Sci. 2013. PMID: 23965975 Free PMC article. Review.
-
The Polyvalent Role of NF90 in RNA Biology.Int J Mol Sci. 2022 Nov 5;23(21):13584. doi: 10.3390/ijms232113584. Int J Mol Sci. 2022. PMID: 36362366 Free PMC article. Review.
Cited by
-
Overexpression of NF90-NF45 Represses Myogenic MicroRNA Biogenesis, Resulting in Development of Skeletal Muscle Atrophy and Centronuclear Muscle Fibers.Mol Cell Biol. 2015 Jul;35(13):2295-308. doi: 10.1128/MCB.01297-14. Epub 2015 Apr 27. Mol Cell Biol. 2015. PMID: 25918244 Free PMC article.
-
Nucleotide composition of cellular internal ribosome entry sites defines dependence on NF45 and predicts a posttranscriptional mitotic regulon.Mol Cell Biol. 2013 Jan;33(2):307-18. doi: 10.1128/MCB.00546-12. Epub 2012 Nov 5. Mol Cell Biol. 2013. PMID: 23129811 Free PMC article.
-
Nuclear Factor 90, a cellular dsRNA binding protein inhibits the HIV Rev-export function.Retrovirology. 2006 Nov 24;3:83. doi: 10.1186/1742-4690-3-83. Retrovirology. 2006. PMID: 17125513 Free PMC article.
-
Long non-coding RNA TPT1-AS1 promotes angiogenesis and metastasis of colorectal cancer through TPT1-AS1/NF90/VEGFA signaling pathway.Aging (Albany NY). 2020 Apr 4;12(7):6191-6205. doi: 10.18632/aging.103016. Epub 2020 Apr 4. Aging (Albany NY). 2020. PMID: 32248186 Free PMC article.
-
Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control.Mol Cell Biol. 2008 Jul;28(14):4629-41. doi: 10.1128/MCB.00120-08. Epub 2008 May 5. Mol Cell Biol. 2008. PMID: 18458058 Free PMC article.
References
-
- Aoki, Y., G. Zhao, D. Qiu, L. Shi, and P. N. Kao. 1998. CsA-sensitive purine-box transcriptional regulator in bronchial epithelial cells contains NF45, NF90, and Ku. Am. J. Physiol. 275:L1164–L1172. - PubMed
-
- Bass, B. L., S. R. Hurst, and J. D. Singer. 1994. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr. Biol. 4:301–314. - PubMed
-
- Buaas, F. W., K. Lee, S. Edelhoff, C. Disteche, and R. E. Braun. 1999. Cloning and characterization of the mouse interleukin enhancer binding factor 3 (Ilf3) homolog in a screen for RNA binding proteins. Mamm. Genome 10:451–456. - PubMed
-
- Carpick, B. W., V. Graziano, D. Schneider, R. K. Maitra, X. Lee, and B. R. Williams. 1997. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 272:9510–9516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
