Identification and characterization of a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors
- PMID: 11739747
- PMCID: PMC134202
- DOI: 10.1128/MCB.22.1.357-369.2002
Identification and characterization of a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors
Abstract
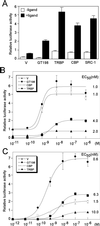
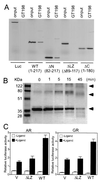
Gene activation mediated by nuclear receptors is regulated in a tissue-specific manner and requires interactions between nuclear receptors and their cofactors. Here, we identified and characterized a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors. GT198 was originally described as a genomic transcript that mapped to the human breast cancer susceptibility locus 17q12-q21 with unknown function. We show that GT198 exhibits a tissue-specific expression pattern in which its mRNA is elevated in testis, spleen, thymus, pituitary cells, and several cancer cell lines. GT198 is a 217-amino-acid nuclear protein that contains a leucine zipper required for its dimerization. In vitro binding and yeast two-hybrid assays indicated that GT198 interacted with nuclear receptors through their DNA-binding domains. GT198 potently stimulated transcription mediated by estrogen receptor alpha and beta, thyroid hormone receptor beta1, androgen receptor, glucocorticoid receptor, and progesterone receptor. However, the action of GT198 was distinguishable from that of the ligand-binding domain-interacting nuclear receptor coactivators, such as TRBP, CBP, and SRC-1, with respect to basal activation and hormone sensitivity. Furthermore, protein kinase A, protein kinase C, and mitogen-activated protein kinase can phosphorylate GT198 in vitro, and cotransfection of these kinases regulated the transcriptional activity of GT198. These data suggest that GT198 is a tissue-specific, kinase-regulated nuclear receptor coactivator that interacts with the DNA-binding domains of nuclear receptors.
Figures



References
-
- Albert, T. 1992. Structure of the leucine zipper. Curr. Opin. Genet. Dev. 2:205–210. - PubMed
-
- Angrand, P.-O., C. Coffinier, and M. C. Weiss. 1994. Response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids depends on the integrity of the cAMP pathway. Cell Growth Differ. 5:957–966. - PubMed
-
- Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968. - PubMed
-
- Aronheim, A. 2000. Protein recruitment systems for the analysis of protein-protein interactions. Biochem. Pharmacol. 60:1009–1013. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
