p19(ARF) is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumor suppression in vivo
- PMID: 11739748
- PMCID: PMC134227
- DOI: 10.1128/MCB.22.1.370-377.2002
p19(ARF) is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumor suppression in vivo
Abstract
Recent studies have shown the p19(ARF) tumor suppressor to be involved in the response to oncogenic stress by regulating the activity of p53. This response is mediated by antagonizing the function of Mdm2, a negative regulator of p53, indicating a pathway for tumor suppression that involves numerous genes altered in human tumors. We previously described a transgenic mouse brain tumor model in which oncogenic stress, provided by cell-specific inactivation of the pRb pathway, triggers a p53-dependent apoptotic response. This response suppresses the growth of developing tumors and thus represents a bona fide in vivo tumor suppressor activity. We further showed that E2F1, a transcription factor known to induce p19(ARF) expression, was required for the response. Here, we use a genetic approach to test whether p19(ARF) functions to transduce the signal from E2F1 to p53 in this tumor suppression pathway. Contrary to the currently accepted hypothesis, we show that a deficiency in p19(ARF) has no impact on p53-mediated apoptosis or tumor suppression in this system. All measures of p53 function, including the level of apoptosis induced by pRb inactivation, the expression of p21 (a p53-responsive gene), and the rate of tumor growth, were comparable in mice with and without a functional p19(ARF) gene. Thus, although p19(ARF) is required in some cell types to transmit an oncogenic response signal to p53, it is dispensable for this function in an in vivo epithelial system. These results underscore the complexity of p53 tumor suppression and further indicate the existence of distinct cell-specific pathways that respond to similar stimuli.
Figures

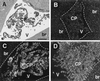
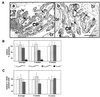
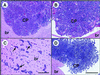
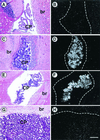
References
-
- Ashcroft, M., and K. H. Vousden. 1999. Regulation of p53 stability. Oncogene 18:7637–7643. - PubMed
-
- Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors RB and p53. Nature 395:124–125. - PubMed
-
- El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
