APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex
- PMID: 11739784
- PMCID: PMC60759
- DOI: 10.1091/mbc.12.12.3839
APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex
Abstract
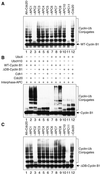
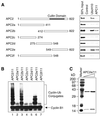
In mitosis, the anaphase-promoting complex (APC) regulates the onset of sister-chromatid separation and exit from mitosis by mediating the ubiquitination and degradation of the securin protein and mitotic cyclins. With the use of a baculoviral expression system, we have reconstituted the ubiquitin ligase activity of human APC. In combination with Ubc4 or UbcH10, a heterodimeric complex of APC2 and APC11 is sufficient to catalyze the ubiquitination of human securin and cyclin B1. However, the minimal APC2/11 ubiquitin ligase module does not possess substrate specificity, because it also ubiquitinates the destruction box deletion mutants of securin and cyclin B1. Both APC11 and UbcH10 bind to the C-terminal cullin homology domain of APC2, whereas Ubc4 interacts with APC11 directly. Zn(2+)-binding and mutagenesis experiments indicate that APC11 binds Zn(2+) at a 1:3 M ratio. Unlike the two Zn(2+) ions of the canonical RING-finger motif, the third Zn(2+) ion of APC11 is not essential for its ligase activity. Surprisingly, with Ubc4 as the E2 enzyme, Zn(2+) ions alone are sufficient to catalyze the ubiquitination of cyclin B1. Therefore, the Zn(2+) ions of the RING finger family of ubiquitin ligases may be directly involved in catalysis.
Figures
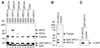
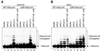



References
-
- Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. - PubMed
-
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. - PubMed
-
- Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

