TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression
- PMID: 11739785
- PMCID: PMC60760
- DOI: 10.1091/mbc.12.12.3852
TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression
Abstract
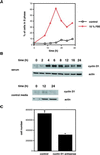
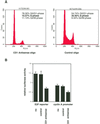
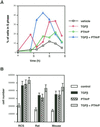
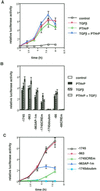
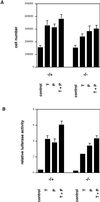
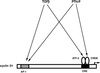
Exact coordination of growth plate chondrocyte proliferation is necessary for normal endochondral bone development and growth. Here we show that PTHrP and TGFbeta control chondrocyte cell cycle progression and proliferation by stimulating signaling pathways that activate transcription from the cyclin D1 promoter. The TGFbeta pathway activates the transcription factor ATF-2, whereas PTHrP uses the related transcription factor CREB, to stimulate cyclin D1 promoter activity via the CRE promoter element. Inhibition of cyclin D1 expression with antisense oligonucleotides causes a delay in progression of chondrocytes through the G1 phase of the cell cycle, reduced E2F activity, and decreased proliferation. Growth plates from cyclin D1-deficient mice display a smaller zone of proliferating chondrocytes, confirming the requirement for cyclin D1 in chondrocyte proliferation in vivo. These data identify the cyclin D1 gene as an essential component of chondrocyte proliferation as well as a fundamental target gene of TGFbeta and PTHrP during skeletal growth.
Figures




Similar articles
-
Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes.Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1433-8. doi: 10.1073/pnas.96.4.1433. Proc Natl Acad Sci U S A. 1999. PMID: 9990041 Free PMC article.
-
The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription.BMC Mol Biol. 2006 Sep 19;7:30. doi: 10.1186/1471-2199-7-30. BMC Mol Biol. 2006. PMID: 16984628 Free PMC article.
-
PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation.J Cell Sci. 2009 May 1;122(Pt 9):1382-9. doi: 10.1242/jcs.040709. Epub 2009 Apr 7. J Cell Sci. 2009. PMID: 19351720 Free PMC article.
-
PTHrP and skeletal development.Ann N Y Acad Sci. 2006 Apr;1068:1-13. doi: 10.1196/annals.1346.002. Ann N Y Acad Sci. 2006. PMID: 16831900 Review.
-
Cell cycle control in growth plate chondrocytes.Front Biosci. 2000 May 1;5:D493-503. doi: 10.2741/luvalle. Front Biosci. 2000. PMID: 10799356 Review.
Cited by
-
ATF2 - at the crossroad of nuclear and cytosolic functions.J Cell Sci. 2012 Jun 15;125(Pt 12):2815-24. doi: 10.1242/jcs.095000. Epub 2012 Jun 8. J Cell Sci. 2012. PMID: 22685333 Free PMC article.
-
Suppressor of fused (Sufu) mediates the effect of parathyroid hormone-like hormone (Pthlh) on chondrocyte differentiation in the growth plate.J Biol Chem. 2012 Oct 19;287(43):36222-8. doi: 10.1074/jbc.M112.382275. Epub 2012 Aug 28. J Biol Chem. 2012. PMID: 22930757 Free PMC article.
-
Effects of acute femoral head ischemia on the growth plate and metaphysis in a piglet model of Legg-Calvé-Perthes disease.Osteoarthritis Cartilage. 2023 Jun;31(6):766-774. doi: 10.1016/j.joca.2023.01.011. Epub 2023 Jan 22. Osteoarthritis Cartilage. 2023. PMID: 36696941 Free PMC article.
-
Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation.Proc Natl Acad Sci U S A. 2006 Jan 10;103(2):365-70. doi: 10.1073/pnas.0507979103. Epub 2005 Dec 30. Proc Natl Acad Sci U S A. 2006. PMID: 16387856 Free PMC article.
-
Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification.Pediatr Nephrol. 2010 Apr;25(4):625-31. doi: 10.1007/s00467-009-1368-6. Epub 2009 Dec 1. Pediatr Nephrol. 2010. PMID: 19949815 Review.
References
-
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. - PubMed
-
- Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

