Dominant-lethal alpha-tubulin mutants defective in microtubule depolymerization in yeast
- PMID: 11739794
- PMCID: PMC60769
- DOI: 10.1091/mbc.12.12.3973
Dominant-lethal alpha-tubulin mutants defective in microtubule depolymerization in yeast
Abstract
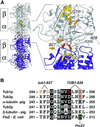
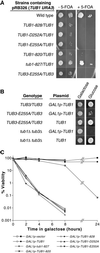
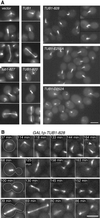
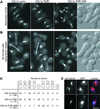
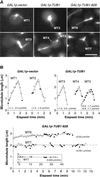
The dynamic instability of microtubules has long been understood to depend on the hydrolysis of GTP bound to beta-tubulin, an event stimulated by polymerization and necessary for depolymerization. Crystallographic studies of tubulin show that GTP is bound by beta-tubulin at the longitudinal dimer-dimer interface and contacts particular alpha-tubulin residues in the next dimer along the protofilament. This structural arrangement suggests that these contacts could account for assembly-stimulated GTP hydrolysis. As a test of this hypothesis, we examined, in yeast cells, the effect of mutating the alpha-tubulin residues predicted, on structural grounds, to be involved in GTPase activation. Mutation of these residues to alanine (i.e., D252A and E255A) created poisonous alpha-tubulins that caused lethality even as minor components of the alpha-tubulin pool. When the mutant alpha-tubulins were expressed from the galactose-inducible promoter of GAL1, cells rapidly acquired aberrant microtubule structures. Cytoplasmic microtubules were largely bundled, spindle assembly was inhibited, preexisting spindles failed to completely elongate, and occasional, stable microtubules were observed unattached to spindle pole bodies. Time-lapse microscopy showed that microtubule dynamics had ceased. Microtubules containing the mutant proteins did not depolymerize, even in the presence of nocodazole. These data support the view that alpha-tubulin is a GTPase-activating protein that acts, during microtubule polymerization, to stimulate GTP hydrolysis in beta-tubulin and thereby account for the dynamic instability of microtubules.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

