Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion
- PMID: 11739798
- PMCID: PMC60773
- DOI: 10.1091/mbc.12.12.4030
Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion
Abstract
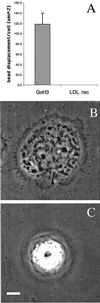
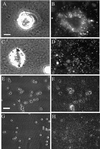
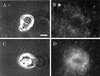
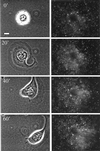
The integrin alpha6beta4, a laminin receptor that stabilizes epithelial cell adhesion to the basement membrane (BM) through its association with cytokeratins, can stimulate the formation and stabilization of actin-rich protrusions in carcinoma cells. An important, unresolved issue, however, is whether this integrin can transmit forces to the substrate generated by the acto-myosin system. Using a traction-force detection assay, we detected forces exerted through alpha6beta4 on either laminin-1 or on an anti-alpha6 antibody, demonstrating that this integrin can transmit forces without the need to engage other integrins. These alpha6beta4-dependent traction forces were organized into a compression machine localized to the base of lamellae. We hypothesized that the compression forces generated by alpha6beta4 result in the remodeling of BMs because this integrin plays a major role in the interaction of epithelial and carcinoma cells with such structures. Indeed, we observed that carcinoma cells are able to remodel a reconstituted BM through alpha6beta4-mediated compression forces by a process that involves the packing of BM material under the cells and the mechanical removal of BM from adjacent areas. The distinct signaling functions of alpha6beta4, which activate phosphoinositide 3-OH kinase and RhoA, also contribute to remodeling. Importantly, we demonstrate remodeling of a native BM by epithelial cells and the involvement of alpha6beta4 in this remodeling. Our findings have important implications for the mechanism of both BM organization and tumor invasion.
Figures






References
-
- Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;216:113–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

