Heterologous production of Clostridium cellulovorans engB, using protease-deficient Bacillus subtilis, and preparation of active recombinant cellulosomes
- PMID: 11741846
- PMCID: PMC134751
- DOI: 10.1128/JB.184.1.76-81.2002
Heterologous production of Clostridium cellulovorans engB, using protease-deficient Bacillus subtilis, and preparation of active recombinant cellulosomes
Abstract
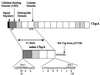
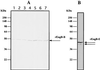
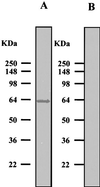
In cellulosomes produced by Clostridium spp., the high-affinity interaction between the dockerin domain and the cohesin domain is responsible for the assembly of enzymatic subunits into the complex. Thus, heterologous expression of full-length enzymatic subunits containing the dockerin domains and of the scaffolding unit is essential for the in vitro assembly of a "designer" cellulosome, or a recombinant cellulosome with a specific function. We report the preparation of Clostridium cellulovorans recombinant cellulosomes containing the enzymatic subunit EngB and the scaffolding unit, mini-CbpA, containing a cellulose binding domain, a putative cell wall binding domain, and two cohesin units. The full-length EngB containing the dockerin domain was expressed by Bacillus subtilis WB800, which is deficient in eight extracellular proteases, to prevent the proteolytic cleavage of the enzymatic subunit between the catalytic and dockerin domains that was observed in previous attempts to express EngB with Escherichia coli. The assembly of recombinant EngB with the mini-CbpA was confirmed by immunostaining, a cellulose binding experiment, and native polyacrylamide gel electrophoresis analysis.
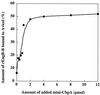
Figures


References
-
- Aminov, R. I., N. P. Golovchenko, and K. Ohmiya. 1995. Expression of a celE Gene from Clostridium thermocellum in Bacillus. J. Ferment. Bioeng. 79:530–537.
-
- Doi, R. H., J. S. Park, C. C. Liu, L. M. Malburg, Y. Tamaru, A. Ichiishi, and A. Ibrahim. 1998. Cellulosome and non-cellulosomal cellulases of Clostridium cellulovorans. Extremophiles 2:53–60. - PubMed
-
- Foong, F., T. Hamamoto, O. Shoseyov, and R. H. Doi. 1991. Nucleotide sequence and characteristics of endoglucanase gene engB from Clostridium cellulovorans. J. Gen. Microbiol. 137:1729–1736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

