Metabolic flux responses to pyruvate kinase knockout in Escherichia coli
- PMID: 11741855
- PMCID: PMC134756
- DOI: 10.1128/JB.184.1.152-164.2002
Metabolic flux responses to pyruvate kinase knockout in Escherichia coli
Abstract
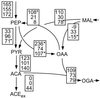
The intracellular carbon flux distribution in wild-type and pyruvate kinase-deficient Escherichia coli was estimated using biosynthetically directed fractional 13C labeling experiments with [U-13C6]glucose in glucose- or ammonia-limited chemostats, two-dimensional nuclear magnetic resonance (NMR) spectroscopy of cellular amino acids, and a comprehensive isotopomer model. The general response to disruption of both pyruvate kinase isoenzymes in E. coli was a local flux rerouting via the combined reactions of phosphoenolpyruvate (PEP) carboxylase and malic enzyme. Responses in the pentose phosphate pathway and the tricarboxylic acid cycle were strongly dependent on the environmental conditions. In addition, high futile cycling activity via the gluconeogenic PEP carboxykinase was identified at a low dilution rate in glucose-limited chemostat culture of pyruvate kinase-deficient E. coli, with a turnover that is comparable to the specific glucose uptake rate. Furthermore, flux analysis in mutant cultures indicates that glucose uptake in E. coli is not catalyzed exclusively by the phosphotransferase system in glucose-limited cultures at a low dilution rate. Reliability of the flux estimates thus obtained was verified by statistical error analysis and by comparison to intracellular carbon flux ratios that were independently calculated from the same NMR data by metabolic flux ratio analysis.
Figures
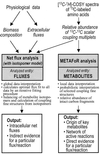
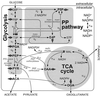


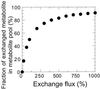
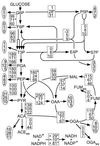

References
-
- Abdel-Hamid, A. M., M. M. Attwood, and J. R. Guest. 2001. Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483–1498. - PubMed
-
- Bäck, T., and H.-P. Schwefel. 1993. An overview of evolutionary algorithms for parameter optimization. Evol. Comp. 1:1–23.
-
- Bergmeyer, J., and M. Grassl. 1983. Enzymes 1: oxidoreductases, transferases. Verlag Chemie, Weinheim, Germany.
-
- Bergmeyer, J., and M. Grassl. 1984. Metabolites 1: carbohydrates. Verlag Chemie, Weinheim, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

