Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli
- PMID: 11741870
- PMCID: PMC134780
- DOI: 10.1128/JB.184.1.290-301.2002
Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli
Abstract
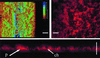
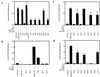
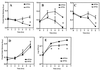
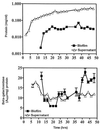
The predominant mode of growth of bacteria in the environment is within sessile, matrix-enclosed communities known as biofilms. Biofilms often complicate chronic and difficult-to-treat infections by protecting bacteria from the immune system, decreasing antibiotic efficacy, and dispersing planktonic cells to distant body sites. While the biology of bacterial biofilms has become a major focus of microbial research, the regulatory mechanisms of biofilm development remain poorly defined and those of dispersal are unknown. Here we establish that the RNA binding global regulatory protein CsrA (carbon storage regulator) of Escherichia coli K-12 serves as both a repressor of biofilm formation and an activator of biofilm dispersal under a variety of culture conditions. Ectopic expression of the E. coli K-12 csrA gene repressed biofilm formation by related bacterial pathogens. A csrA knockout mutation enhanced biofilm formation in E. coli strains that were defective for extracellular, surface, or regulatory factors previously implicated in biofilm formation. In contrast, this csrA mutation did not affect biofilm formation by a glgA (glycogen synthase) knockout mutant. Complementation studies with glg genes provided further genetic evidence that the effects of CsrA on biofilm formation are mediated largely through the regulation of intracellular glycogen biosynthesis and catabolism. Finally, the expression of a chromosomally encoded csrA'-'lacZ translational fusion was dynamically regulated during biofilm formation in a pattern consistent with its role as a repressor. We propose that global regulation of central carbon flux by CsrA is an extremely important feature of E. coli biofilm development.
Figures




References
-
- Allison, D. G., B. Ruiz, C. San Jose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179–184. - PubMed
-
- Applegate, D. H., and J. D. Bryers. 1991. Effects of carbon and oxygen limitations and calcium concentrations on biofilm removal processes. Biotech. Bioeng. 37:17–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

