Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase
- PMID: 11742092
- PMCID: PMC64948
- DOI: 10.1073/pnas.261469998
Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase
Abstract
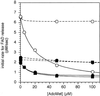
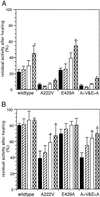
Methylenetetrahydrofolate reductase (MTHFR) catalyzes the conversion of methylenetetrahydrofolate to methyltetrahydrofolate, the major methyl donor for the conversion of homocysteine to methionine. Two common polymorphisms of the human enzyme have been identified: 677C>T, which leads to the substitution of Ala-222 by valine, and 1298A>C, which leads to the replacement of Glu-429 by alanine; the former polymorphism is the most frequent genetic cause of mild hyperhomocysteinemia, a risk factor for cardiovascular disease. By using a baculovirus expression system, recombinant human MTHFR has been expressed at high levels and purified to homogeneity in quantities suitable for biochemical characterization. The Glu429Ala protein has biochemical properties that are indistinguishable from the wild-type enzyme. The Ala222Val MTHFR, however, has an enhanced propensity to dissociate into monomers and to lose its FAD cofactor on dilution; the resulting loss of activity is slowed in the presence of methyltetrahydrofolate or adenosylmethionine. This biochemical phenotype is in good agreement with predictions made on the basis of studies comparing wild-type Escherichia coli MTHFR with a mutant, Ala177Val, homologous to the Ala222Val mutant human enzyme [Guenther, B. D., et al. (1999) Nat. Struct. Biol. 6, 359-365].
Figures



Comment in
-
Genetic diversity and disease: opportunities and challenge.Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):14754-6. doi: 10.1073/pnas.011601398. Proc Natl Acad Sci U S A. 2001. PMID: 11752418 Free PMC article. No abstract available.
References
-
- Boushey C J, Beresford S A A, Omenn G S, Motulsky A G. J Am Med Assn. 1995;274:1049–1057. - PubMed
-
- Graham I M, Daly L E, Refsum H M, Robinson K, Brattstrom L E, Ueland P M, Palma-Reis J, Boers G H J, Sheahan R G, Israelsson B, et al. J Am Med Assn. 1997;277:1775–1781. - PubMed
-
- Refsum H, Ueland P M, Nygard O, Vollset S E. Annu Rev Med. 1998;49:31–62. - PubMed
-
- Chen Z, Karaplis A C, Ackerman S L, Pobribny I P, Melnyk S, Lussier-Cacan S, Chen M F, Pai A, John S W M, Smith R S, et al. Hum Mol Genet. 2001;10:433–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

