Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection
- PMID: 11742096
- PMCID: PMC65023
- DOI: 10.1073/pnas.261560998
Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection
Abstract
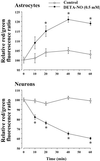
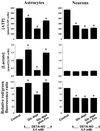
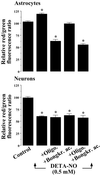
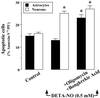
It was recently proposed that in Jurkat cells, after inhibition of respiration by NO, glycolytically generated ATP plays a critical role in preventing the collapse of mitochondrial membrane potential (Deltapsi(m)) and thus apoptotic cell death. We have investigated this observation further in primary cultures of rat cortical neurons and astrocytes-cell types that differ greatly in their glycolytic capacity. Continuous and significant ( approximately 85%) inhibition of respiration by NO (1.4 microM at 175 microM O(2)) generated by [(z)-1-[2-aminoethyl]-N-[2-ammonioethyl]amino]diazen-1-ium-1,2 diolate (DETA-NO) initially (10 min) depleted ATP concentrations by approximately 25% in both cell types and increased the rate of glycolysis in astrocytes but not in neurons. Activation of glycolysis in astrocytes, as judged by lactate production, prevented further ATP depletion, whereas in neurons, which do not invoke this mechanism, there was a progressive decrease in ATP concentrations over the next 60 min. During this time, there was a persistent mitochondrial hyperpolarization and absence of apoptotic cell death in astrocytes, whereas in the neurons there was a progressive fall in Deltapsi(m) and increased apoptosis. After glucose deprivation or treatment with inhibitors of the F(1)F(0)-ATPase and adenine nucleotide translocase, astrocytes responded to NO with a fall in Deltapsi(m) and apoptotic cell death similar to the response in neurons. Finally, although treatment of astrocytes with NO partially prevented staurosporin-induced collapse in Deltapsi(m) and cell death, NO and staurosporin synergized in decreasing Deltapsi(m) and inducing apoptosis in neurons. These results demonstrate that although inhibition of cellular respiration by NO leads to neurotoxicity, it may also result in initial neuroprotection, depending on the glycolytic capacity of the particular cell.
Figures

References
-
- Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. - PubMed
-
- Melino G, Bernassola F, Catani M V, Rossi A, Corazzari M, Sabatini S, Vilbois F, Green D R. Cancer Res. 2000;60:2377–2383. - PubMed
-
- Sastry P S, Rao K S. J Neurochem. 2000;74:1–20. - PubMed
-
- Mitchell P. Nature (London) 1961;191:144–148. - PubMed
-
- Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

