Subunit interaction maps for the regulatory particle of the 26S proteasome and the COP9 signalosome
- PMID: 11742986
- PMCID: PMC125776
- DOI: 10.1093/emboj/20.24.7096
Subunit interaction maps for the regulatory particle of the 26S proteasome and the COP9 signalosome
Abstract
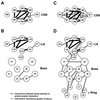
The 26S proteasome plays a major role in eukaryotic protein breakdown, especially for ubiquitin-tagged proteins. Substrate specificity is conferred by the regulatory particle (RP), which can dissociate into stable lid and base subcomplexes. To help define the molecular organization of the RP, we tested all possible paired interactions among subunits from Saccharomyces cerevisiae by yeast two-hybrid analysis. Within the base, a Rpt4/5/3/6 interaction cluster was evident. Within the lid, a structural cluster formed around Rpn5/11/9/8. Interactions were detected among synonymous subunits (Csn4/5/7/6) from the evolutionarily related COP9 signalosome (CSN) from Arabidopsis, implying a similar quaternary arrangement. No paired interactions were detected between lid, base or core particle subcomplexes, suggesting that stable contacts between them require prior assembly. Mutational analysis defined the ATPase, coiled-coil, PCI and MPN domains as important for RP assembly. A single residue in the vWA domain of Rpn10 is essential for amino acid analog resistance, for degrading a ubiquitin fusion degradation substrate and for stabilizing lid-base association. Comprehensive subunit interaction maps for the 26S proteasome and CSN support the ancestral relationship of these two complexes.
Figures



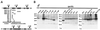

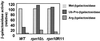

References
-
- Deng X.W. et al. (2000) Unified nomenclature for the COP9 signalosome and its subunits: an essential regulator of development. Trends Genet., 16, 202–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

