Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators
- PMID: 11742989
- PMCID: PMC125339
- DOI: 10.1093/emboj/20.24.7128
Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators
Abstract
The circadian timing system in mammals is composed of a master pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus and slave clocks in most peripheral cell types. The phase of peripheral clocks can be completely uncoupled from the SCN pacemaker by restricted feeding. Thus, feeding time, while not affecting the phase of the SCN pacemaker, is a dominant Zeitgeber for peripheral circadian oscillators. Here we show that the phase resetting in peripheral clocks of nocturnal mice is slow when feeding time is changed from night to day and rapid when switched back from day to night. Unexpectedly, the inertia in daytime feeding-induced phase resetting of circadian gene expression in liver and kidney is not an intrinsic property of peripheral oscillators, but is caused by glucocorticoid signaling. Thus, glucocorticoid hormones inhibit the uncoupling of peripheral and central circadian oscillators by altered feeding time.
Figures

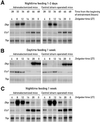



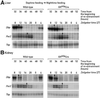

References
-
- Albrecht U., Sun,Z.S., Eichele,G. and Lee,C.C. (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell, 91, 1055–1064. - PubMed
-
- Balsalobre A., Damiola,F. and Schibler,U. (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell, 93, 929–937. - PubMed
-
- Balsalobre A., Brown,S.A., Marcacci,L., Tronche,F., Kellendonk,C., Reichardt,H.M., Schutz,G. and Schibler,U. (2000a) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289, 2344–2347. - PubMed
-
- Balsalobre A., Marcacci,L. and Schibler,U. (2000b) Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol., 10, 1291–1294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

