Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine
- PMID: 11744753
- PMCID: PMC2279010
- DOI: 10.1111/j.1469-7793.2001.00763.x
Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine
Abstract
1. Nucleotide activation of skeletal muscle ryanodine receptors (RyRs) was studied in planar lipid bilayers in order to understand RyR regulation in vivo under normal and fatigued conditions. With 'resting' calcium (100 nM cytoplasmic and 1 mM luminal), RyRs had an open probability (P(o)) of approximately 0.01 in the absence of nucleotides and magnesium. ATP reversibly activated RyRs with P(o) at saturation (P(max)) approximately 0.33 and K(a) (concentration for half-maximal activation) approximately 0.36 mM and with a Hill coefficient (n(H)) of approximately 1.8 in RyRs when P(max) < 0.5 and approximately 4 when P(max) > 0.5. 2. AMP was a much weaker agonist (P(max) approximately 0.09) and adenosine was weaker still (P(max) approximately 0.01-0.02), whereas inosine monophosphate (IMP), the normal metabolic end product of ATP hydrolysis, produced no activation at all. 3. Adenosine acted as a competitive antagonist that reversibly inhibited ATP- and AMP-activated RyRs with n(H) approximately 1 and K(i) approximately 0.06 mM at [ATP] < 0.5 mM, increasing 4-fold for each 2-fold increase in [ATP] above 0.5 mM. This is explained by the binding of a single adenosine preventing the cooperative binding of two ATP or AMP molecules, with dissociation constants of 0.4, 0.45 and 0.06 mM for ATP, AMP and adenosine, respectively. Importantly, IMP (< or = 8 mM) had no inhibitory effect whatsoever on ATP-activated RyRs. 4. Mean open (tau(o)) and closed (tau(c)) dwell-times were more closely related to P(o) than to the nucleotide species or individual RyRs. At P(o) < 0.2, RyR regulation occurred via changes in tau(c), whereas at higher P(o) this also occurred via changes in tau(o). The detailed properties of activation and competitive inhibition indicated complex channel behaviour that could be explained in terms of a model involving interactions between different subunits of the RyR homotetramer. 5. The results also show how deleterious adenosine accumulation is to the function of RyRs in skeletal muscle and, by comparison with voltage sensor-controlled Ca(2+) release, indicate that voltage sensor activation requires ATP binding to the RyR to be effective.
Figures


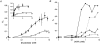






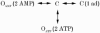
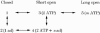
References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. - PubMed
-
- Blazev R, Lamb GD. Adenosine inhibits depolarization-induced Ca2+ release in mammalian skeletal muscle. Muscle and Nerve. 1999b;22:1674–1683. - PubMed
-
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Annals of Biochemistry. 1992;201:119–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

