Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells
- PMID: 11744761
- PMCID: PMC2279013
- DOI: 10.1111/j.1469-7793.2001.00871.x
Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells
Abstract
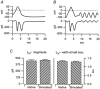
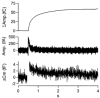
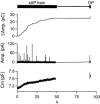
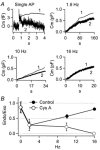
1. Exocytosis and endocytosis were measured following single, or trains of, simulated action potentials (sAP) in bovine adrenal chromaffin cells. Catecholamine secretion was measured by oxidative amperometry and cell membrane turnover was measured by voltage clamp cell capacitance measurements. 2. The sAPs evoked inward Na(+) and Ca(2+) currents that were statistically identical to those evoked by native action potential waveforms. On average, a single secretory granule underwent fusion following sAP stimulation. An equivalent amount of membrane was then quickly internalised (tau = 560 ms). 3. Stimulation with sAP trains revealed a biphasic relationship between cell firing rate and endocytic activity. At basal stimulus frequencies (single to 0.5 Hz) cells exhibited a robust membrane internalisation that then diminished as firing increased to intermediate levels (1.9 and 6 Hz). However at the higher stimulation rates (10 and 16 Hz) endocytic activity rebounded and was again able to effectively maintain cell surface near pre-stimulus levels. 4. Treatment with cyclosporin A and FK506, inhibitors of the phosphatase calcineurin, left endocytosis characteristics unaltered at the lower basal stimulus levels, but blocked the resurgence in endocytosis seen in control cells at higher sAP frequencies. 5. Based on these findings we propose that, under physiological electrical stimulation, chromaffin cells internalise membrane via two distinct pathways that are separable. One is prevalent at basal stimulus frequencies, is lessened with increased firing, and is insensitive to cyclosporin A and FK506. A second endocytic form is activated by increased firing frequencies, and is selectively blocked by cyclosporin A and FK506.
Figures




References
-
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. - PubMed
-
- Artalejo CR, Elhamdani A, Palfrey HC. Secretion: dense-core vesicles can kiss-and-run too. Current Biology. 1998;8:R62–65. - PubMed
-
- Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. International Review of Cytology. 1998;181:213–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

