T-tubule localization of the inward-rectifier K(+) channel in mouse ventricular myocytes: a role in K(+) accumulation
- PMID: 11744770
- PMCID: PMC2278989
- DOI: 10.1111/j.1469-7793.2001.00979.x
T-tubule localization of the inward-rectifier K(+) channel in mouse ventricular myocytes: a role in K(+) accumulation
Abstract
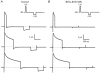
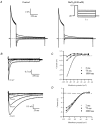
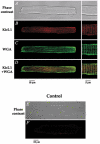
1. The properties of the slow inward 'tail currents' (I(tail)) that followed depolarizing steps in voltage-clamped, isolated mouse ventricular myocytes were examined. Depolarizing steps that produced large outward K(+) currents in these myocytes were followed by a slowly decaying inward I(tail) on repolarization to the holding potential. These currents were produced only by depolarizations: inwardly rectifying K(+) currents, I(K1), produced by steps to potentials negative to the holding potential, were not followed by I(tail). 2. For depolarizations of equal duration, the magnitude of I(tail) increased as the magnitude of outward current at the end of the depolarizing step increased. The apparent reversal potential of I(tail) was dependent upon the duration of the depolarizing step, and the reversal potential shifted to more depolarized potentials as the duration of the depolarization was increased. 3. Removal of external Na(+) and Ca(2+) had no significant effect on the magnitude or time course of I(tail). BaCl(2) (0.25 mM), which had no effect on the magnitude of outward currents, abolished I(tail) and I(K1) simultaneously. 4. Accordingly, I(tail) in mouse ventricular myocytes probably results from K(+) accumulation in a restricted extracellular space such as the transverse tubule system (t-tubules). The efflux of K(+) into the t-tubules during outward currents produced by depolarization shifts the K(+) Nernst potential (E(K)) from its 'resting' value (close to -80 mV) to more depolarized potentials. This suggests that I(tail) is produced by I(K1) in the t-tubules and is inward because of the transiently elevated K(+) concentration and depolarized value of E(K) in the t-tubules. 5. Additional evidence for the localization of I(K1) channels in the t-tubules was provided by confocal microscopy using a specific antibody against Kir2.1 in mouse ventricular myocytes.
Figures






Similar articles
-
Potassium currents in adult rat intracardiac neurones.J Physiol. 1995 Jul 1;486 ( Pt 1)(Pt 1):15-31. doi: 10.1113/jphysiol.1995.sp020787. J Physiol. 1995. PMID: 7562632 Free PMC article.
-
Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture.J Physiol. 1994 Sep 1;479 ( Pt 2)(Pt 2):265-79. doi: 10.1113/jphysiol.1994.sp020294. J Physiol. 1994. PMID: 7799226 Free PMC article.
-
Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current.Circ Res. 2004 May 28;94(10):1332-9. doi: 10.1161/01.RES.0000128408.66946.67. Epub 2004 Apr 15. Circ Res. 2004. PMID: 15087421
-
Cardiac strong inward rectifier potassium channels.J Mol Cell Cardiol. 2010 Jan;48(1):45-54. doi: 10.1016/j.yjmcc.2009.08.013. Epub 2009 Aug 22. J Mol Cell Cardiol. 2010. PMID: 19703462 Free PMC article. Review.
-
Inward rectifier potassium channels control rotor frequency in ventricular fibrillation.Heart Rhythm. 2009 Nov;6(11 Suppl):S44-8. doi: 10.1016/j.hrthm.2009.07.019. Epub 2009 Sep 1. Heart Rhythm. 2009. PMID: 19880073 Free PMC article. Review.
Cited by
-
Transmural heterogeneity of repolarization and Ca2+ handling in a model of mouse ventricular tissue.Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H454-69. doi: 10.1152/ajpheart.00907.2009. Epub 2010 Jun 4. Am J Physiol Heart Circ Physiol. 2010. PMID: 20525874 Free PMC article.
-
Voltage dependence of ATP-dependent K+ current in rat cardiac myocytes is affected by IK1 and IK(ACh).J Physiol. 2004 Dec 1;561(Pt 2):459-69. doi: 10.1113/jphysiol.2004.073197. Epub 2004 Sep 30. J Physiol. 2004. PMID: 15459245 Free PMC article.
-
Rigorous Phenotyping of Cardiac iPSC Preparations Requires Knowledge of Their Resting Potential(s).Biophys J. 2016 Jan 5;110(1):278-80. doi: 10.1016/j.bpj.2015.06.070. Biophys J. 2016. PMID: 26745430 Free PMC article. No abstract available.
-
MicroRNA Biophysically Modulates Cardiac Action Potential by Direct Binding to Ion Channel.Circulation. 2021 Apr 20;143(16):1597-1613. doi: 10.1161/CIRCULATIONAHA.120.050098. Epub 2021 Feb 16. Circulation. 2021. PMID: 33590773 Free PMC article.
-
Diffusional and Electrical Properties of T-Tubules Are Governed by Their Constrictions and Dilations.Biophys J. 2018 Jan 23;114(2):437-449. doi: 10.1016/j.bpj.2017.11.3742. Biophys J. 2018. PMID: 29401441 Free PMC article.
References
-
- Attwell DE, Cohen IS. The voltage clamp of multicellular preparations. Progress in Biophysics and Molecular Biology. 1977;31:201–245. - PubMed
-
- Attwell DE, Eisner DA, Cohen IS. Voltage clamp and tracer flux data: effects of a restricted extracellular space. Quarterly Review of Biophysics. 1979;12:213–263. - PubMed
-
- Bers DM, Lederer WJ, Berlin JR. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. American Journal of Physiology. 1990;258:C944–954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

