Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics
- PMID: 11747900
- PMCID: PMC2855216
- DOI: 10.1016/s0028-3908(01)00141-1
Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics
Abstract
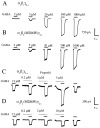
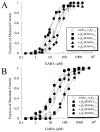
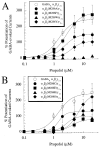
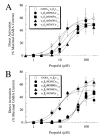
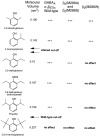
gamma-Aminobutyric acid type A (GABA(A)) receptors are an important target for general anesthetics in the central nervous system. Site-directed mutagenesis techniques have identified amino acid residues that are important for the positive modulation of GABA(A) receptors by general anesthetics. In the present study, we investigate the role of an amino acid residue in transmembrane (TM) domain 3 of the GABA(A) receptor beta(2) subunit for modulation by the general anesthetic 2,6-diisopropylphenol (propofol). Mutation of methionine 286 to tryptophan (M286W) in the beta(2) subunit abolished potentiation of GABA responses by propofol but did not affect direct receptor activation by propofol in the absence of GABA. In contrast, substitution of methionine 286 by alanine, cysteine, glutamate, lysine, phenylalanine, serine, or tyrosine was permissive for potentiation of GABA responses and direct activation by propofol. Using propofol analogs of varying molecular size, we show that the beta(2)(M286W) mutation resulted in a decrease in the 'cut-off' volume for propofol analog molecules to enhance GABA responses at GABA(A) alpha(1)beta(2)gamma(2s) receptors. This suggests that mutation of M286 in the GABA(A) beta(2) subunit alters the dimensions of a 'binding pocket' for propofol and related alkylphenol general anesthetics.
Figures
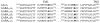

Similar articles
-
Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type A receptor distinct from that for isoflurane.Mol Pharmacol. 1998 Mar;53(3):530-8. doi: 10.1124/mol.53.3.530. Mol Pharmacol. 1998. PMID: 9495821
-
A single glycine residue at the entrance to the first membrane-spanning domain of the gamma-aminobutyric acid type A receptor beta(2) subunit affects allosteric sensitivity to GABA and anesthetics.Mol Pharmacol. 2000 Mar;57(3):474-84. doi: 10.1124/mol.57.3.474. Mol Pharmacol. 2000. PMID: 10692487
-
Tryptophan mutations at azi-etomidate photo-incorporation sites on alpha1 or beta2 subunits enhance GABAA receptor gating and reduce etomidate modulation.Mol Pharmacol. 2008 Dec;74(6):1687-95. doi: 10.1124/mol.108.050500. Epub 2008 Sep 19. Mol Pharmacol. 2008. PMID: 18805938 Free PMC article.
-
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation.Can J Anaesth. 2011 Feb;58(2):206-15. doi: 10.1007/s12630-010-9429-7. Epub 2010 Dec 31. Can J Anaesth. 2011. PMID: 21194017 Free PMC article. Review.
-
Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABAA Receptors by Propofol.Curr Neuropharmacol. 2016;14(7):772-80. doi: 10.2174/1570159x14666160202121319. Curr Neuropharmacol. 2016. PMID: 26830963 Free PMC article. Review.
Cited by
-
Postoperative expressive aphasia associated with intravenous midazolam administration: a 5-year retrospective case-control study.J Int Med Res. 2020 Aug;48(8):300060520948751. doi: 10.1177/0300060520948751. J Int Med Res. 2020. PMID: 32851907 Free PMC article.
-
Shared structural mechanisms of general anaesthetics and benzodiazepines.Nature. 2020 Sep;585(7824):303-308. doi: 10.1038/s41586-020-2654-5. Epub 2020 Sep 2. Nature. 2020. PMID: 32879488 Free PMC article.
-
Channel opening by anesthetics and GABA induces similar changes in the GABAA receptor M2 segment.Biophys J. 2007 May 1;92(9):3130-9. doi: 10.1529/biophysj.106.094490. Epub 2007 Feb 9. Biophys J. 2007. PMID: 17293408 Free PMC article.
-
p-(4-Azipentyl)propofol: a potent photoreactive general anesthetic derivative of propofol.J Med Chem. 2011 Dec 8;54(23):8124-35. doi: 10.1021/jm200943f. Epub 2011 Nov 10. J Med Chem. 2011. PMID: 22029276 Free PMC article.
-
Analysis of γ-aminobutyric acid (GABA) type A receptor subtypes using isosteric and allosteric ligands.Neurochem Res. 2014 Oct;39(10):1924-41. doi: 10.1007/s11064-014-1382-3. Epub 2014 Jul 12. Neurochem Res. 2014. PMID: 25015397
References
-
- Amin J. A single hydrophobic residue confers barbiturate sensitivity to γ-aminobutyric acid type C receptor. Molecular Pharmacology. 1999;55:411–423. - PubMed
-
- Bai D, Zhu G, Pennefather PS, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Molecular Pharmacology. 2001;59:814–824. - PubMed
-
- Belelli D, Pistis M, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends in Pharmacological Sciences. 1999;20:496–502. - PubMed
-
- Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin: high-resolution crystal structures. Journal of Biological Chemistry. 2000;275:38731–38738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

