Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity
- PMID: 11748170
- PMCID: PMC127632
- DOI: 10.1128/IAI.70.1.107-113.2002
Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity
Abstract
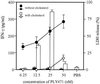
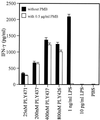
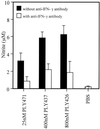
Pneumolysin (PLY), an important virulence factor of Streptococcus pneumoniae, is known to exert various effects on the host immune cells, including cytokine induction, in addition to its known cytolytic activity as a member of the thiol-activated cytolysins. It is of interest to determine whether cytolytic activity is involved in triggering the cytokine production. In this study, we constructed full-length recombinant PLY and noncytolytic truncated PLYs with C-terminal deletions to examine the response of spleen cells to these PLY preparations. When cytolytic activity was blocked by treatment with cholesterol, full-length PLY was capable of inducing gamma interferon (IFN-gamma) production. Truncated PLYs that originally exhibited no cytolytic activity were also active in IFN-gamma induction. Therefore, the IFN-gamma-inducing ability of PLY appeared to be independent of the cytolytic activity. Furthermore, IFN-gamma-inducing preparations were also capable of inducing nitric oxide synthase expression and nitric oxide (NO) production, and the addition of neutralizing antibody to IFN-gamma abolished the NO production. These results clearly demonstrated that PLY is capable of inducing IFN-gamma production in spleen cells by a mechanism different from pore formation and that the induced IFN-gamma stimulates NO production. These findings were discussed with reference to the contribution of PLY to the virulence of S. pneumoniae in vivo.
Figures


References
-
- Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24: 167–174 - PubMed
-
- Alouf, J. E. 1999. Introduction to the family of the structurally related cholesterol-binding cytolysins (‘sulfhydryl-activated’ toxins), p. 443–456. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, England
-
- Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2001. Essential role of domain 4 of pneumolysin from Streptococcus pneumoniae in cytolytic activity as determined by truncated proteins. Biochem. Biophys. Res. Commun. 281: 37–44 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

