The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells
- PMID: 11748175
- PMCID: PMC127600
- DOI: 10.1128/IAI.70.1.140-146.2002
The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells
Abstract
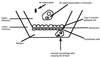
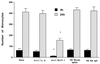
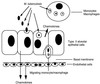
The mechanism(s) by which Mycobacterium tuberculosis crosses the alveolar wall to establish infection in the lung is not well known. In an attempt to better understand the mechanism of translocation and create a model to study the different stages of bacterial crossing through the alveolar wall, we established a two-layer transwell system. M. tuberculosis H37Rv was evaluated regarding the ability to cross and disrupt the membrane. M. tuberculosis invaded A549 type II alveolar cells with an efficiency of 2 to 3% of the initial inoculum, although it was not efficient in invading endothelial cells. However, bacteria that invaded A549 cells were subsequently able to be taken up by endothelial cells with an efficiency of 5 to 6% of the inoculum. When incubated with a bicellular transwell monolayer (epithelial and endothelial cells), M. tuberculosis translocated into the lower chamber with efficiency (3 to 4%). M. tuberculosis was also able to efficiently translocate across the bicellular layer when inside monocytes. Infected monocytes crossed the barrier with greater efficiency when A549 alveolar cells were infected with M. tuberculosis than when A549 cells were not infected. We identified two potential mechanisms by which M. tuberculosis gains access to deeper tissues, by translocating across epithelial cells and by traveling into the blood vessels within monocytes.
Figures
References
-
- Bermudez, L. E. 1993. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J. Immunol. 150: 1838–1845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

