Dependence of Mycobacterium bovis BCG on anaerobic nitrate reductase for persistence is tissue specific
- PMID: 11748194
- PMCID: PMC127612
- DOI: 10.1128/IAI.70.1.286-291.2002
Dependence of Mycobacterium bovis BCG on anaerobic nitrate reductase for persistence is tissue specific
Abstract
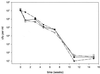
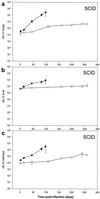
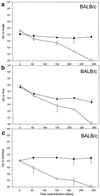
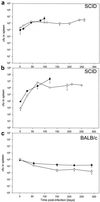
Mycobacterium bovis BCG, the only presently available vaccine against tuberculosis, was obtained from virulent M. bovis after serial passages in vitro. The vaccine strain retained at least some of its original virulence, as it persists in immune-competent hosts and occasionally may cause fatal disease in immune-deficient hosts. Mycobacterial persistence in vivo is thought to depend on anaerobic metabolism, an apparent paradox since all mycobacteria are obligate aerobes. Here we report that M. bovis BCG lacking anaerobic nitrate reductase (NarGHJI), an enzyme essential for nitrate respiration, failed to persist in the lungs, liver, and kidneys of immune-competent (BALB/c) mice. In immune-deficient (SCID) mice, however, bacilli caused chronic infection despite disruption of narG, even if growth of the mutant was severely impaired in lungs, liver, and kidneys. Persistence and growth of BCG in the spleens of either mouse strain appeared largely unaffected by lack of anaerobic nitrate reductase, indicating that the role of the enzyme in pathogenesis is tissue specific. These data suggest first that anaerobic nitrate reduction is essential for metabolism of M. bovis BCG in immune-competent but not immune-deficient mice and second that its role in mycobacterial disease is tissue specific, both of which are observations with important implications for pathogenesis of mycobacteria and vaccine development.
Figures

References
-
- Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351: 479–482. - PubMed
-
- Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280: 1432–1435. - PubMed
-
- Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Doffinger, S. Lamhamedi, P. Drysdale, D. Scheel-Toellner, J. Girdlestone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102: 2035–2040. - PMC - PubMed
-
- Barclay, R., and P. R. Wheeler. 1989. Metabolism of mycobacteria in tissues, p. 37–106. In C. Ratledge, J. Stanford, and J. M. Grange (ed.), Clinical aspects of mycobacterial disease. Academic Press, London, United Kingdom.
-
- Blasco, F., C. Iobbi, G. Giordano, M. Chippaux, and V. Bonnefoy. 1989. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the alpha and beta subunits in iron binding and electron transfer. Mol. Gen. Genet. 218: 249–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

