Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells
- PMID: 11748197
- PMCID: PMC127624
- DOI: 10.1128/IAI.70.1.315-322.2002
Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells
Abstract
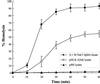
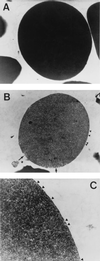
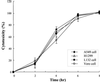
Leptospirosis is a spirochetal zoonosis that causes an acute febrile systemic illness in humans. Leptospira sp. hemolysins have been shown to be virulence factors for the pathogenesis of leptospirosis. Previously, we cloned a hemolysin SphH of Leptospira interrogans serovar lai, a homologue of L. borgpetersenii sphingomyelinase (SphA), from a genomic library (S. H. Lee, K. A. Kim, Y. K. Kim, I. W. Seong, M. J. Kim, and Y. J. Lee, Gene 254:19-28, 2000). Escherichia coli lysate harboring the sphH showed high hemolytic activities on sheep erythrocytes. However, it neither showed sphingomyelinase nor phospholipase activities, in contrast to SphA which was known to have sphingomyelinase activity. Interestingly, the SphH-mediated hemolysis on erythrocytes was osmotically protected by PEG 5000, suggesting that the SphH might have caused pore formation on the erythrocyte membrane. In the present study, we have prepared the Leptospira hemolysin SphH and investigated its hemolytic and cytotoxic activities on mammalian cells. SphH was shown to be a pore-forming protein on several mammalian cells: When treated with the SphH, the sheep erythrocyte membranes formed pores, which were morphologically confirmed by transmission electron microscopy. Furthermore, the SphH-mediated cytotoxicities on mammalian cells were demonstrated by the release of LDH and by inverted microscopic examinations. Finally, the immune serum against the full-length hemolysin could effectively neutralize the SphH-mediated hemolytic and cytotoxic activities. In conclusion, these results suggest that the virulence of Leptospira SphH was due to the pore formation on mammalian cell membranes.
Figures



Similar articles
-
Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar lai.Gene. 2000 Aug 22;254(1-2):19-28. doi: 10.1016/s0378-1119(00)00293-6. Gene. 2000. PMID: 10974532
-
Identification and classification of all potential hemolysin encoding genes and their products from Leptospira interrogans serogroup Icterohae-morrhagiae serovar Lai.Acta Pharmacol Sin. 2005 Apr;26(4):453-61. doi: 10.1111/j.1745-7254.2005.00075.x. Acta Pharmacol Sin. 2005. PMID: 15780195
-
Role of sph2 Gene Regulation in Hemolytic and Sphingomyelinase Activities Produced by Leptospira interrogans.PLoS Negl Trop Dis. 2015 Aug 14;9(8):e0003952. doi: 10.1371/journal.pntd.0003952. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26274394 Free PMC article.
-
Serratia type pore forming toxins.Curr Protein Pept Sci. 2000 Jul;1(1):75-89. doi: 10.2174/1389203003381423. Curr Protein Pept Sci. 2000. PMID: 12369921 Review.
-
Pathogenesis of leptospirosis: the influence of genomics.Vet Microbiol. 2011 Nov 21;153(1-2):73-81. doi: 10.1016/j.vetmic.2011.02.055. Epub 2011 Mar 5. Vet Microbiol. 2011. PMID: 21440384 Review.
Cited by
-
In silico prediction of molecular mechanisms of toxicity mediated by the leptospiral PF07598 gene family-encoded virulence-modifying proteins.Front Mol Biosci. 2023 Jan 23;9:1092197. doi: 10.3389/fmolb.2022.1092197. eCollection 2022. Front Mol Biosci. 2023. PMID: 36756251 Free PMC article.
-
Extracellular secretion of the Borrelia burgdorferi Oms28 porin and Bgp, a glycosaminoglycan binding protein.Infect Immun. 2004 Nov;72(11):6279-86. doi: 10.1128/IAI.72.11.6279-6286.2004. Infect Immun. 2004. PMID: 15501754 Free PMC article.
-
Leptospirosis vaccines.Microb Cell Fact. 2007 Dec 11;6:39. doi: 10.1186/1475-2859-6-39. Microb Cell Fact. 2007. PMID: 18072968 Free PMC article.
-
Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily.Mol Microbiol. 2003 Aug;49(4):929-45. doi: 10.1046/j.1365-2958.2003.03619.x. Mol Microbiol. 2003. PMID: 12890019 Free PMC article.
-
Transcriptional responses of Leptospira interrogans to host innate immunity: significant changes in metabolism, oxygen tolerance, and outer membrane.PLoS Negl Trop Dis. 2010 Oct 26;4(10):e857. doi: 10.1371/journal.pntd.0000857. PLoS Negl Trop Dis. 2010. PMID: 21049008 Free PMC article.
References
-
- Alves, V. A., L. C. Gayotto, T. De Brito, R. T. Santos, A. Wakamatsu, M. R. Vianna, and E. E. Sakata. 1992. Leptospiral antigens in the liver of experimentally infected guinea pig and their relation to the morphogenesis of liver damage. Exp. Toxicol. Pathol. 44: 425–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

