Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans
- PMID: 11748199
- PMCID: PMC127630
- DOI: 10.1128/IAI.70.1.335-344.2002
Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans
Abstract
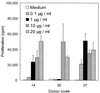
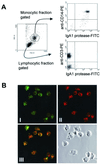
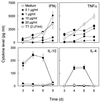
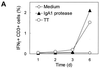
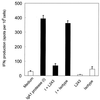
We have previously shown that immunoglobulin A1 (IgA1) protease, an exoenzyme of pathogenic neisseriae, can trigger the release of proinflammatory cytokines from human monocytic subpopulations. Here, we demonstrate a dose-dependent T-cell response to recombinant gonococcal IgA1 protease (strain MS11) in healthy human blood donors. This response was delayed in comparison to the immune response against tetanus toxoid. Stimulation with IgA1 protease led to the activation of CD4(+) and CD8(+) T cells, as well as CD19(+) B cells and CD56(+) NK cells, indicated by de novo expression of CD69. Only CD4(+) T cells proliferated and stained positive for intracellular gamma interferon (IFN-gamma). Both proliferation and IFN-gamma production were dependent on antigen presentation via major histocompatibility complex class II. Peripheral blood mononuclear cells stimulated with IgA1 protease produce IFN-gamma and tumor necrosis factor alpha but no, or very low amounts of, interleukin-10 (IL-10) or IL-4, indicating a Th1-based proinflammatory immune response. These findings support the significance of IgA1 protease as a virulence determinant of bacterial meningitis and its function as a dominant proinflammatory T-cell antigen.
Figures




Similar articles
-
Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines.J Exp Med. 1999 Oct 18;190(8):1049-58. doi: 10.1084/jem.190.8.1049. J Exp Med. 1999. PMID: 10523603 Free PMC article.
-
Lipopolysaccharide stimulates the proliferation of human CD56+CD3- NK cells: a regulatory role of monocytes and IL-10.J Immunol. 2000 Jul 1;165(1):139-47. doi: 10.4049/jimmunol.165.1.139. J Immunol. 2000. PMID: 10861046
-
CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon-gamma and tumour necrosis factor-alpha in response to the recombinant 14KDA Schistosoma mansoni fatty acid-binding protein.Scand J Immunol. 2000 Jun;51(6):595-601. doi: 10.1046/j.1365-3083.2000.00710.x. Scand J Immunol. 2000. PMID: 10849370
-
Activation of human T cells with NK cell markers by staphylococcal enterotoxin A via IL-12 but not via IL-18.Clin Exp Immunol. 2002 Jun;128(3):453-9. doi: 10.1046/j.1365-2249.2002.01854.x. Clin Exp Immunol. 2002. PMID: 12109440 Free PMC article.
-
Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells.Immunology. 2001 Jul;103(3):281-90. doi: 10.1046/j.1365-2567.2001.01248.x. Immunology. 2001. PMID: 11454057 Free PMC article.
Cited by
-
Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells.Immunology. 2004 Jul;112(3):428-36. doi: 10.1111/j.1365-2567.2004.01898.x. Immunology. 2004. PMID: 15196211 Free PMC article.
-
Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells.Immunology. 2006 Jun;118(2):164-70. doi: 10.1111/j.1365-2567.2006.02347.x. Immunology. 2006. PMID: 16771851 Free PMC article.
-
Opa+ and Opa- isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells.Infect Immun. 2009 Nov;77(11):5170-80. doi: 10.1128/IAI.00355-09. Epub 2009 Aug 31. Infect Immun. 2009. PMID: 19720754 Free PMC article.
-
Human Immune Responses and the Natural History of Neisseria gonorrhoeae Infection.Front Immunol. 2019 Feb 19;9:3187. doi: 10.3389/fimmu.2018.03187. eCollection 2018. Front Immunol. 2019. PMID: 30838004 Free PMC article. Review.
-
Characterization of igaB, a second immunoglobulin A1 protease gene in nontypeable Haemophilus influenzae.Infect Immun. 2006 Oct;74(10):5860-70. doi: 10.1128/IAI.00796-06. Infect Immun. 2006. PMID: 16988265 Free PMC article.
References
-
- Ait-Tahar, K., K. G. Wooldridge, D. P. Turner, M. Atta, I. Todd, and D. A. Ala’Aldeen. 2000. Auto-transporter A protein of Neisseria meningitidis: a potent CD4+ T-cell and B-cell stimulating antigen detected by expression cloning. Mol. Microbiol. 37: 1094–1105. - PubMed
-
- Bass, J. L., R. Nuss, K. A. Mehta, P. Morganelli, and L. Bennett. 1983. Recurrent meningococcemia associated with IgG2-subclass deficiency. N. Engl. J. Med. 309: 430. - PubMed
-
- Beck, S. C., and T. F. Meyer. 2000. IgA1 protease from Neisseria gonorrhoeae inhibits TNF alpha-mediated apoptosis of human monocytic cells. FEBS Lett. 472: 287–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

