Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis
- PMID: 11748209
- PMCID: PMC127647
- DOI: 10.1128/IAI.70.1.407-411.2002
Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis
Abstract
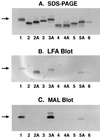
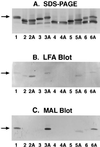
The sialylation of lipooligosaccharide (LOS) in Neisseria meningitidis plays a role in the resistance of the organism to killing by normal human serum. The length of the alpha chain extending out from the heptose I [Hep (I)] moiety of LOS influenced sialylation of N. meningitidis LOS in vitro and in vivo. The alpha chain required a terminal Gal and a trisaccharide or longer oligosaccharide to serve as an acceptor for sialylation. The disaccharide lactose (Galbeta1-4Glc) in the alpha chain of immunotype L8 LOS could not function as an acceptor for the sialyltransferase, probably due to steric hindrance imposed by the neighboring Hep (II) with phosphorylethanolamine and another group attached.
Figures
Similar articles
-
α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract.mBio. 2015 Feb 3;6(1):e02465-14. doi: 10.1128/mBio.02465-14. mBio. 2015. PMID: 25650401 Free PMC article.
-
The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea.mBio. 2021 Mar 23;12(2):e03666-20. doi: 10.1128/mBio.03666-20. mBio. 2021. PMID: 33758087 Free PMC article.
-
The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein.Infect Immun. 2002 Jul;70(7):3744-51. doi: 10.1128/IAI.70.7.3744-3751.2002. Infect Immun. 2002. PMID: 12065517 Free PMC article.
-
Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity.Microb Pathog. 1995 Dec;19(6):365-77. doi: 10.1006/mpat.1995.0071. Microb Pathog. 1995. PMID: 8852278 Review. No abstract available.
-
Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galbeta1-4GlcNAcbeta1-3Galbeta1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins.Adv Exp Med Biol. 2001;491:525-42. doi: 10.1007/978-1-4615-1267-7_35. Adv Exp Med Biol. 2001. PMID: 14533820 Review.
Cited by
-
Profiles of structural heterogeneity in native lipooligosaccharides of Neisseria and cytokine induction.J Lipid Res. 2009 Mar;50(3):424-438. doi: 10.1194/jlr.M800184-JLR200. Epub 2008 Oct 2. J Lipid Res. 2009. PMID: 18832773 Free PMC article.
-
Genetic and structural characterization of L11 lipooligosaccharide from Neisseria meningitidis serogroup A strains.J Biol Chem. 2010 Jun 25;285(26):19874-83. doi: 10.1074/jbc.M110.100636. Epub 2010 Apr 26. J Biol Chem. 2010. PMID: 20421293 Free PMC article.
-
Lipooligosaccharide Structures of Invasive and Carrier Isolates of Neisseria meningitidis Are Correlated with Pathogenicity and Carriage.J Biol Chem. 2016 Feb 12;291(7):3224-38. doi: 10.1074/jbc.M115.666214. Epub 2015 Dec 11. J Biol Chem. 2016. PMID: 26655715 Free PMC article.
-
Sweet impersonators: Molecular mimicry of host glycans by bacteria.Glycobiology. 2022 Feb 26;32(1):11-22. doi: 10.1093/glycob/cwab104. Glycobiology. 2022. PMID: 34939094 Free PMC article. Review.
-
Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains.Infect Immun. 2003 Jul;71(7):3901-8. doi: 10.1128/IAI.71.7.3901-3908.2003. Infect Immun. 2003. PMID: 12819075 Free PMC article.
References
-
- Apicella, M. A., R. E. Mandrell, M. Shero, et al. 1990. Modification of sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162: 506–512. - PubMed
-
- Estabrook, M. M., N. C. Christopher, J. M. Griffiss, C. J. Baker, and R. E. Mandrell. 1992. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J. Infect. Dis. 166: 1079–1088. - PubMed
-
- Gilbert, M., A.-M. Cunningham, D. C. Watson, A. Martin, C. J. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis α-2,3-sialyltransferase and its acceptor affinity. Eur. J. Biochem. 249: 187–194. - PubMed
-
- Gotschlich, E. C. 1990. Neisseriae, p. 551–560. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 4th ed. J. B. Lippincott, Philadelphia, Pa.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

