Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis
- PMID: 11748209
- PMCID: PMC127647
- DOI: 10.1128/IAI.70.1.407-411.2002
Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis
Abstract
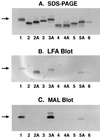
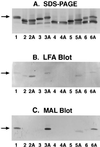
The sialylation of lipooligosaccharide (LOS) in Neisseria meningitidis plays a role in the resistance of the organism to killing by normal human serum. The length of the alpha chain extending out from the heptose I [Hep (I)] moiety of LOS influenced sialylation of N. meningitidis LOS in vitro and in vivo. The alpha chain required a terminal Gal and a trisaccharide or longer oligosaccharide to serve as an acceptor for sialylation. The disaccharide lactose (Galbeta1-4Glc) in the alpha chain of immunotype L8 LOS could not function as an acceptor for the sialyltransferase, probably due to steric hindrance imposed by the neighboring Hep (II) with phosphorylethanolamine and another group attached.
Figures
References
-
- Apicella, M. A., R. E. Mandrell, M. Shero, et al. 1990. Modification of sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162: 506–512. - PubMed
-
- Estabrook, M. M., N. C. Christopher, J. M. Griffiss, C. J. Baker, and R. E. Mandrell. 1992. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J. Infect. Dis. 166: 1079–1088. - PubMed
-
- Gilbert, M., A.-M. Cunningham, D. C. Watson, A. Martin, C. J. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis α-2,3-sialyltransferase and its acceptor affinity. Eur. J. Biochem. 249: 187–194. - PubMed
-
- Gotschlich, E. C. 1990. Neisseriae, p. 551–560. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 4th ed. J. B. Lippincott, Philadelphia, Pa.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

