Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae
- PMID: 11748210
- PMCID: PMC127640
- DOI: 10.1128/IAI.70.1.412-415.2002
Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae
Abstract
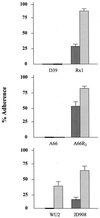
Nonspecific interactions related to physicochemical properties of bacterial cell surfaces, such as hydrophobicity and electrostatic charge, are known to have important roles in bacterium-host cell encounters. Streptococcus pneumoniae (pneumococcus) expresses multiple, surface-exposed, choline-binding proteins (CBPs) which have been associated with adhesion and virulence. The purpose of this study was to determine the contribution of CBPs to the surface characteristics of pneumococci and, consequently, to learn how CBPs may affect nonspecific interactions with host cells. Pneumococcal strains lacking CBPs were derived by adapting bacteria to a defined medium that substituted ethanolamine for choline. Such strains do not anchor CBPs to their surface. Cell surface hydrophobicity was tested for the wild-type and adapted strains by using a biphasic hydrocarbon adherence assay, and electrostatic charge was determined by zeta potential measurement. Adherence of pneumococci to human-derived cells was assessed by fluorescence-activated cell sorter analysis. Strains lacking both capsule and CBPs were significantly more hydrophobic than nonencapsulated strains with a normal complement of CBPs. The effect of CBPs on hydrophobicity was attenuated in the presence of capsule. Removal of CBPs conferred a greater electronegative net surface charge than that which cells with CBPs possessed, regardless of the presence of capsule. Strains that lack CBPs were poorly adherent to human monocyte-like cells when compared with wild-type bacteria with a full complement of CBPs. These results suggest that CBPs contribute significantly to the hydrophobic and electrostatic surface characteristics of pneumococci and may facilitate adherence to host cells partially through nonspecific, physicochemical interactions.
Figures
References
-
- Ausubel, F., R. Brent, R. E. Kingston, et al (ed.). 1993. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Beachey, E. H. (ed.). 1980. General concepts and priniciples of bacterial adherence in animals and man, vol. 6. Chapman & Hall, London, United Kingdom.
-
- Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14: 858–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

