Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans
- PMID: 11748251
- PMCID: PMC2199344
- DOI: 10.1083/jcb.200108051
Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans
Abstract
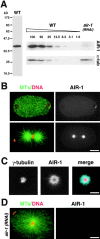
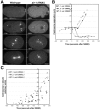
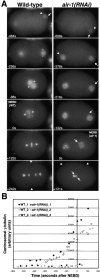
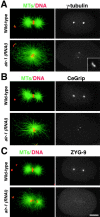
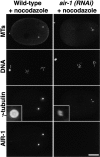
Centrosomes mature as cells enter mitosis, accumulating gamma-tubulin and other pericentriolar material (PCM) components. This occurs concomitant with an increase in the number of centrosomally organized microtubules (MTs). Here, we use RNA-mediated interference (RNAi) to examine the role of the aurora-A kinase, AIR-1, during centrosome maturation in Caenorhabditis elegans. In air-1(RNAi) embryos, centrosomes separate normally, an event that occurs before maturation in C. elegans. After nuclear envelope breakdown, the separated centrosomes collapse together, and spindle assembly fails. In mitotic air-1(RNAi) embryos, centrosomal alpha-tubulin fluorescence intensity accumulates to only 40% of wild-type levels, suggesting a defect in the maturation process. Consistent with this hypothesis, we find that AIR-1 is required for the increase in centrosomal gamma-tubulin and two other PCM components, ZYG-9 and CeGrip, as embryos enter mitosis. Furthermore, the AIR-1-dependent increase in centrosomal gamma-tubulin does not require MTs. These results suggest that aurora-A kinases are required to execute a MT-independent pathway for the recruitment of PCM during centrosome maturation.
Figures
Similar articles
-
The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent.J Cell Biol. 2002 May 13;157(4):591-602. doi: 10.1083/jcb.200202047. Epub 2002 May 13. J Cell Biol. 2002. PMID: 12011109 Free PMC article.
-
Centrosomes promote timely mitotic entry in C. elegans embryos.Dev Cell. 2007 Apr;12(4):531-41. doi: 10.1016/j.devcel.2007.02.015. Dev Cell. 2007. PMID: 17419992
-
The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis.Curr Biol. 2008 Jan 22;18(2):136-41. doi: 10.1016/j.cub.2007.12.055. Curr Biol. 2008. PMID: 18207742
-
Polar expeditions--provisioning the centrosome for mitosis.Nat Cell Biol. 2003 Jun;5(6):505-11. doi: 10.1038/ncb0603-505. Nat Cell Biol. 2003. PMID: 12776127 Review.
-
On the role of aurora-A in centrosome function.Oncogene. 2002 Sep 9;21(40):6175-83. doi: 10.1038/sj.onc.1205775. Oncogene. 2002. PMID: 12214247 Review.
Cited by
-
Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material.Nat Cell Biol. 2012 Nov;14(11):1148-58. doi: 10.1038/ncb2591. Epub 2012 Oct 21. Nat Cell Biol. 2012. PMID: 23086237
-
Expression of constitutively active CDK1 stabilizes APC-Cdh1 substrates and potentiates premature spindle assembly and checkpoint function in G1 cells.PLoS One. 2012;7(3):e33835. doi: 10.1371/journal.pone.0033835. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479455 Free PMC article.
-
High KDM1A Expression Associated with Decreased CD8+T Cells Reduces the Breast Cancer Survival Rate in Patients with Breast Cancer.J Clin Med. 2021 Mar 7;10(5):1112. doi: 10.3390/jcm10051112. J Clin Med. 2021. PMID: 33799951 Free PMC article.
-
Aurora Kinases as Therapeutic Targets in Head and Neck Cancer.Cancer J. 2022 Sep-Oct 01;28(5):387-400. doi: 10.1097/PPO.0000000000000614. Cancer J. 2022. PMID: 36165728 Free PMC article.
-
Clathrin promotes centrosome integrity in early mitosis through stabilization of centrosomal ch-TOG.J Cell Biol. 2012 Aug 20;198(4):591-605. doi: 10.1083/jcb.201205116. Epub 2012 Aug 13. J Cell Biol. 2012. PMID: 22891263 Free PMC article.
References
-
- Bischoff, J.R., and G.D. Plowman. 1999. The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9:454–459. - PubMed
-
- Callaini, G., W.G. Whitfield, and M.G. Riparbelli. 1997. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp. Cell Res. 234:183–190. - PubMed
-
- Fry, A.M., T. Mayor, and E.A. Nigg. 2000. Regulating centrosomes by protein phosphorylation. Curr. Top. Dev. Biol. 49:291–312. - PubMed
-
- Giet, R., and C. Prigent. 1999. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell Sci. 112:3591–3601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

