Proteasomal regulation of betac signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization
- PMID: 11748263
- PMCID: PMC209471
- DOI: 10.1172/JCI13877
Proteasomal regulation of betac signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization
Abstract
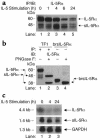
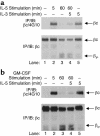
IL-5, IL-3, and GM-CSF are hematopoietic cytokines that are key mediators of the allergic inflammatory response. The receptors for these three cytokines consist of a cytokine-specific alpha (Ralpha) chain and a shared common beta (betac) chain. Herein, we demonstrate that agonistic ligation of these receptor subunits rapidly induces proteasomal degradation of the betac, but not the Ralpha, cytoplasmic domain, resulting in termination of signal transduction and yielding a truncated betac isoform ligated to the Ralpha subunit. Proteasomal degradation of the betac cytoplasmic domain was also a prerequisite for endocytosis and lysosomal degradation of the ligated receptor subunits. Moreover, proteasome-dependent termination of signaling induced by one betac-engaging cytokine resulted in cellular desensitization to signal transduction by subsequent stimulation with another betac-engaging cytokine. These data provide the first evidence for ligand-dependent proteasomal degradation of the betac cytoplasmic domain, and they establish a novel mechanism for heterotypic desensitization of shared cytokine receptor signaling.
Figures
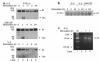




References
-
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993;82:3515–3523. - PubMed
-
- Arai KI, et al. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. - PubMed
-
- Tavernier J, et al. A human high affinity interleukin-5 receptor (IL-5R) is composed of an IL-5-specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1184. - PubMed
-
- Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared β subunit for the human IL-3 and GM-CSF receptors. Cell. 1991;66:1165–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

