Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis
- PMID: 11748266
- PMCID: PMC209462
- DOI: 10.1172/JCI12097
Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis
Abstract
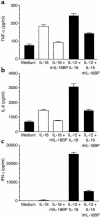
Two distinct IL-18 neutralizing strategies, i.e. a rabbit polyclonal anti-mouse IL-18 IgG and a recombinant human IL-18 binding protein (rhIL-18BP), were used to treat collagen-induced-arthritic DBA/1 mice after clinical onset of disease. The therapeutic efficacy of neutralizing endogenous IL-18 was assessed using different pathological parameters of disease progression. The clinical severity in mice undergoing collagen-induced arthritis was significantly reduced after treatment with both IL-18 neutralizing agents compared to placebo treated mice. Attenuation of the disease was associated with reduced cartilage erosion evident on histology. The decreased cartilage degradation was further documented by a significant reduction in the levels of circulating cartilage oligomeric matrix protein (an indicator of cartilage turnover). Both strategies efficiently slowed disease progression, but only anti-IL-18 IgG treatment significantly decreased an established synovitis. Serum levels of IL-6 were significantly reduced with both neutralizing strategies. In vitro, neutralizing IL-18 resulted in a significant inhibition of TNF-alpha, IL-6, and IFN-gamma secretion by macrophages. These results demonstrate that neutralizing endogenous IL-18 is therapeutically efficacious in the murine model of collagen-induced arthritis. IL-18 neutralizing antibody or rhIL-18BP could therefore represent new disease-modifying anti-rheumatic drugs that warrant testing in clinical trials in patients with rheumatoid arthritis.
Figures





Similar articles
-
Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion.Arthritis Rheum. 2004 Feb;50(2):650-9. doi: 10.1002/art.20001. Arthritis Rheum. 2004. PMID: 14872510
-
Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein.Arthritis Rheum. 2000 Dec;43(12):2668-77. doi: 10.1002/1529-0131(200012)43:12<2668::AID-ANR6>3.0.CO;2-E. Arthritis Rheum. 2000. PMID: 11145024
-
Adenoviral delivery of IL-18 binding protein C ameliorates collagen-induced arthritis in mice.Gene Ther. 2003 Jun;10(12):1004-11. doi: 10.1038/sj.gt.3301986. Gene Ther. 2003. PMID: 12776157
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
-
Collagen-induced arthritis in mice: a major role for tumor necrosis factor-alpha.Methods Mol Biol. 2007;361:265-84. doi: 10.1385/1-59745-208-4:265. Methods Mol Biol. 2007. PMID: 17172717 Review.
Cited by
-
Flare-up of cytokines in rheumatoid arthritis and their role in triggering depression: Shared common function and their possible applications in treatment (Review).Biomed Rep. 2021 Jan;14(1):16. doi: 10.3892/br.2020.1392. Epub 2020 Nov 19. Biomed Rep. 2021. PMID: 33269077 Free PMC article. Review.
-
Treatment of IL-18-binding protein biologics suppresses fibrotic progression in metabolic dysfunction-associated steatohepatitis.Cell Rep Med. 2025 Apr 15;6(4):102047. doi: 10.1016/j.xcrm.2025.102047. Cell Rep Med. 2025. PMID: 40239621 Free PMC article.
-
Targeting cytokines beyond tumor necrosis factor-alpha and interleukin-1 in rheumatoid arthritis.Curr Rheumatol Rep. 2004 Oct;6(5):336-42. doi: 10.1007/s11926-004-0007-2. Curr Rheumatol Rep. 2004. PMID: 15355745 Review.
-
Interleukin-18 as an in vivo mediator of monocyte recruitment in rodent models of rheumatoid arthritis.Arthritis Res Ther. 2010;12(3):R118. doi: 10.1186/ar3055. Epub 2010 Jun 16. Arthritis Res Ther. 2010. PMID: 20565717 Free PMC article.
-
Inflammasomes and the IL-1 Family in Bone Homeostasis and Disease.Curr Osteoporos Rep. 2022 Jun;20(3):170-185. doi: 10.1007/s11914-022-00729-8. Epub 2022 May 14. Curr Osteoporos Rep. 2022. PMID: 35567665 Free PMC article. Review.
References
-
- Ushio S, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. - PubMed
-
- Kohno K, et al. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. - PubMed
-
- Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

