Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease
- PMID: 11748267
- PMCID: PMC209465
- DOI: 10.1172/JCI13116
Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease
Abstract
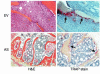
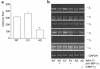
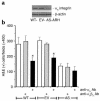
We recently identified macrophage inflammatory protein 1-alpha (MIP-1alpha) as a factor produced by multiple myeloma (MM) cells that may be responsible for the bone destruction in MM (1). To investigate the role of MIP-1alpha in MM bone disease in vivo, the human MM-derived cell line ARH was stably transfected with an antisense construct to MIP-1alpha (AS-ARH) and tested for its capacity to induce MM bone disease in SCID mice. Human MIP-1alpha levels in marrow plasma from AS-ARH mice were markedly decreased compared with controls treated with ARH cells transfected with empty vector (EV-ARH). Mice treated with AS-ARH cells lived longer than controls and, unlike the controls, they showed no radiologically identifiable lytic lesions. Histomorphometric analysis demonstrated that osteoclasts (OCLs) per square millimeter of bone and OCLs per millimeter of bone surface of AS-ARH mice were significantly less than in EV-ARH mice, and the percentage of tumors per total bone area was also significantly decreased. AS-ARH cells demonstrated decreased adherence to marrow stromal cells, due to reduced expression of the alpha(5)beta(1) integrin and diminished homing capacity and survival. These data support an important role for MIP-1alpha in cell homing, survival, and bone destruction in MM.
Figures

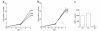





References
-
- Alsina M, et al. Development of an in vivo model of human multiple myeloma bone disease. Blood. 1996;87:1495–1501. - PubMed
-
- Choi SJ, et al. Macrophage inflammatory protein 1-alpha (MIP-1α) is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. - PubMed
-
- Han JH, Choi SJ, Kurihara N, Oba Y, Roodman GD. Macrophage inflammatory protein 1-alpha is an osteoclastogenic factor in myeloma that is independent of RANK ligand. Blood. 2001;97:3349–3353. - PubMed
-
- Kukita T, et al. Macrophage inflammatory protein-1α (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab Invest. 1997;76:399–406. - PubMed
-
- Fuller K, Owens JM, Chambers TJ. Macrophage inflammatory protein-1-alpha and IL-18 stimulate the motility but suppress the resorption of isolated rat osteoclasts. J Immunol. 1995;154:6065–6072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

