Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3
- PMID: 11748272
- PMCID: PMC2193583
- DOI: 10.1084/jem.194.12.1699
Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3
Abstract
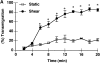
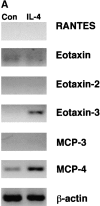
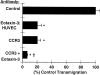
Leukocyte infiltration into inflammatory sites is regulated by the expression of adhesion and activation proteins, yet the role of these proteins in shear-dependent transmigration is poorly understood. We examined eosinophil recruitment on cytokine-stimulated human umbilical vein endothelial cells (HUVECs) under laminar flow conditions. Eosinophils rapidly transmigrated on interleukin (IL)-4-, but not TNF-stimulated HUVECs. Transmigration was shear dependent, with up to 90% of eosinophils transmigrating in the presence of shear and less than 25% of cells transmigrating under static conditions. Eosinophils express CC chemokine receptor CCR3 and are responsive to various CC chemokines. The effects of chemokines are mediated primarily through G(alpha)i, which is pertussis toxin sensitive. Greater than 65% of shear-dependent eosinophil transmigration on IL-4-stimulated HUVECs was blocked by either pertussis toxin or by an anti-CCR3 monoclonal antibody. Using reverse transcription polymerase chain reaction (RT-PCR) and Western blots, we found that IL-4-stimulated HUVECs produce both mRNA and protein for eotaxin-3. Eotaxin-3 was both released by HUVECs and expressed on the endothelial cell surface. Pretreatment of HUVECs with an anti-eotaxin-3 antibody blocked eosinophil transmigration to the same extent as an anti-CCR3 antibody. These results indicate that IL-4-stimulated HUVECs support shear-dependent eosinophil transmigration by upregulating eotaxin-3, and that surface association is critical for the role of eotaxin-3 in transmigration.
Figures







Similar articles
-
Migration of eosinophils across endothelial cell monolayers: interactions among IL-5, endothelial-activating cytokines, and C-C chemokines.J Immunol. 2000 Apr 1;164(7):3847-54. doi: 10.4049/jimmunol.164.7.3847. J Immunol. 2000. PMID: 10725746
-
A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils.J Immunol. 1999 Aug 1;163(3):1602-10. J Immunol. 1999. PMID: 10415065
-
CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2.J Leukoc Biol. 2013 Aug;94(2):213-22. doi: 10.1189/jlb.0212074. Epub 2013 Mar 26. J Leukoc Biol. 2013. PMID: 23532518
-
Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutical implications.Curr Drug Targets Inflamm Allergy. 2003 Mar;2(1):81-94. doi: 10.2174/1568010033344480. Curr Drug Targets Inflamm Allergy. 2003. PMID: 14561178 Review.
-
Eotaxin and asthma.Curr Opin Pharmacol. 2001 Jun;1(3):248-53. doi: 10.1016/s1471-4892(01)00044-3. Curr Opin Pharmacol. 2001. PMID: 11712747 Review.
Cited by
-
Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress.Biophys J. 2004 Sep;87(3):2035-42. doi: 10.1529/biophysj.104.042192. Biophys J. 2004. PMID: 15345579 Free PMC article.
-
Pathological role of large intestinal IL-12p40 for the induction of Th2-type allergic diarrhea.Am J Pathol. 2004 Apr;164(4):1327-35. doi: 10.1016/S0002-9440(10)63219-1. Am J Pathol. 2004. PMID: 15039220 Free PMC article.
-
CXCL12-induced monocyte-endothelial interactions promote lymphocyte transmigration across an in vitro blood-brain barrier.Sci Transl Med. 2012 Feb 1;4(119):119ra14. doi: 10.1126/scitranslmed.3003197. Sci Transl Med. 2012. PMID: 22301555 Free PMC article.
-
Rho GTPases and leucocyte-induced endothelial remodelling.Biochem J. 2005 Jan 15;385(Pt 2):329-37. doi: 10.1042/BJ20041584. Biochem J. 2005. PMID: 15496138 Free PMC article. Review.
-
An endothelial microRNA-1-regulated network controls eosinophil trafficking in asthma and chronic rhinosinusitis.J Allergy Clin Immunol. 2020 Feb;145(2):550-562. doi: 10.1016/j.jaci.2019.10.031. J Allergy Clin Immunol. 2020. PMID: 32035607 Free PMC article.
References
-
- Frigas, E., S. Motojima, and G.J. Gleich. 1991. The eosinophilic injury to the mucosa of the airways in the pathogenesis of bronchial asthma. Eur. Respir. J. (Suppl. 13):123s–135s. - PubMed
-
- Gleich, G.J. 1990. The eosinophil and bronchial asthma: current understanding. J. Allergy Clin. Immunol. 85:422–436. - PubMed
-
- Wardlaw, A.J., R. Moqbel, and A.B. Kay. 1995. Eosinophils: biology and role in disease. Adv. Immunol. 60:151–266. - PubMed
-
- Gopalan, P.K., A.R. Burns, S.I. Simon, S. Sparks, L.V. McIntire, and C.W. Smith. 2000. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J. Leukoc. Biol. 68:47–57. - PubMed

