Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology
- PMID: 11748285
- PMCID: PMC2193571
- DOI: 10.1084/jem.194.12.1847
Alphabeta T cell receptor-positive cells and interferon-gamma, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology
Abstract
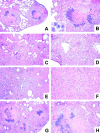
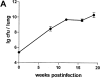
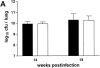
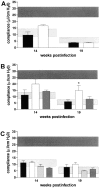
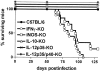
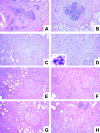
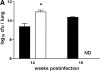
The immunological basis of tuberculin-induced necrosis, known for more than a century as "Koch's phenomenon," remains poorly understood. Aerosol infection in mice with the highly virulent Mycobacterium avium strain TMC724 causes progressive pulmonary pathology strongly resembling caseating necrosis in human patients with tuberculosis. To identify the cellular and molecular mediators causing this pathology, we infected C57BL/6 mice and mice selectively deficient in recombinase activating gene (RAG)-1, alphabeta T cell receptor (TCR), gammadelta TCR, CD4, CD8, beta2-microglobulin, interferon (IFN)-gamma, interleukin (IL)-10, IL-12p35, IL-12p35/p40, or iNOS with M. avium by aerosol and compared bacterial multiplication, histopathology, and respiratory physiology in these mice. The bacterial load in the lung was similarly high in all mouse groups. Pulmonary compliance, as a surrogate marker for granulomatous infiltrations in the lung, deteriorated to a similar extent in all groups of mice, except in alphabeta TCR-knockout (KO) and IL-12-KO mice in which compliance was higher, and in IFN-gamma and inducible nitric oxide synthase-KO mice in which compliance was reduced faster. Progressive caseation of pulmonary granulomas never occurred in alphabeta TCR-KO, IL-12-KO, and IFN-gamma-KO mice and was reduced in CD4-KO mice. In summary, alphabeta TCR(+) cells and IFN-gamma are essential for the development of mycobacteria-induced pulmonary caseous necrosis. In contrast, high mycobacterial load and extensive granulomatous infiltration per se are not sufficient to cause caseation, nor is granuloma necrosis linked to the induction of nitric oxide.
Figures













References
-
- Dannenberg, A.M., Jr. 1991. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol. Today. 12:228–233. - PubMed
-
- Cotran, R.S., V. Kumar, and S.L. Robbins. 1994. Inflammation and repair. Robbins Pathologic Basis of Disease. R.S. Cotran, S. Robbins, and V. Kumar, editors. W.B. Saunders Company, Philadelphia, PA. pp. 51–92.
-
- Ehlers, S. 1999. Immunity to tuberculosis: a delicate balance between protection and pathology. FEMS Immunol. Med. Microbiol. 23:149–158. - PubMed
-
- Dannenberg, A.M., Jr. 1999. Pathophysiology: basic aspects. Tuberculosis and Nontuberculous Mycobacterial Infections. D. Schlossberg, editor. W.B. Saunders Company, Philadelphia, PA. pp. 17–47.
-
- Jagirdar, J., and D. Zagzag. 1996. Pathology and insights into pathogenesis of tuberculosis. Tuberculosis. W.N. Rom, and S. Garay, editors. Little, Brown, and Co., Boston, MA. pp. 467–482.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

