In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis
- PMID: 11751108
- PMCID: PMC126975
- DOI: 10.1128/AAC.46.1.34-41.2002
In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis
Abstract
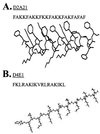
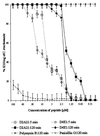
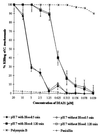
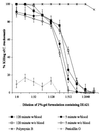
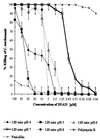
Topically applied microbicides that eradicate pathogens at the time of initial exposure represent a powerful strategy for the prevention of sexually transmitted infections. To aid in the further development of an effective topical microbicide, we assessed the minimum cidal concentration (MCC) of two cecropin peptides, D2A21 and D4E1, and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis in vitro. The MCC of peptide D2A21 was 5 microM (18.32 microg/ml), and that of peptide D4E1 was 7.5 microM (21.69 microg/ml). The MCC of gel formulations containing 2% D2A21 was 0.2 mM (0.7 mg/ml), and that of gel formulations containing 0.5% D2A21 was 0.2 mM (0.7 mg/ml). There was no significant variation in the results when two different C. trachomatis strains were tested, and the addition of 10% human blood did not significantly alter the MCCs. pH values above and below 7 reduced the activity of the D2A21 peptide alone, but the MCC of the 2% D2A21 gel formulation was only slightly altered at the various pHs tested. Ultrastructural studies indicated that C. trachomatis membranes were disrupted after D2A21 exposure, resulting in leakage of the cytoplasmic contents. These in vitro results suggest that these cecropin peptides may be an effective topical microbicide against C. trachomatis and support the need for further evaluation.
Figures
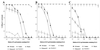
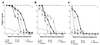

Similar articles
-
Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk.Antimicrob Agents Chemother. 1998 May;42(5):1239-44. doi: 10.1128/AAC.42.5.1239. Antimicrob Agents Chemother. 1998. PMID: 9593157 Free PMC article.
-
Evaluation of WLBU2 peptide and 3-O-octyl-sn-glycerol lipid as active ingredients for a topical microbicide formulation targeting Chlamydia trachomatis.Antimicrob Agents Chemother. 2010 Feb;54(2):627-36. doi: 10.1128/AAC.00635-09. Epub 2009 Dec 14. Antimicrob Agents Chemother. 2010. PMID: 20008784 Free PMC article.
-
Chlamydia trachomatis laboratory strains versus recent clinical isolates: implications for routine microbicide testing.Antimicrob Agents Chemother. 2009 Apr;53(4):1482-9. doi: 10.1128/AAC.01179-08. Epub 2009 Feb 2. Antimicrob Agents Chemother. 2009. PMID: 19188383 Free PMC article.
-
Host defense peptides: general overview and an update on their activity against Chlamydia spp.Expert Rev Anti Infect Ther. 2013 Nov;11(11):1215-24. doi: 10.1586/14787210.2013.841450. Epub 2013 Oct 10. Expert Rev Anti Infect Ther. 2013. PMID: 24111488 Review.
-
Molecular mechanisms of Chlamydia trachomatis resistance to antimicrobial drugs.Front Biosci (Landmark Ed). 2018 Jan 1;23(4):656-670. doi: 10.2741/4611. Front Biosci (Landmark Ed). 2018. PMID: 28930567 Review.
Cited by
-
Natural products for the treatment of trachoma and Chlamydia trachomatis.Molecules. 2015 Mar 5;20(3):4180-203. doi: 10.3390/molecules20034180. Molecules. 2015. PMID: 25751782 Free PMC article. Review.
-
Natural Products for the Treatment of Chlamydiaceae Infections.Microorganisms. 2016 Oct 16;4(4):39. doi: 10.3390/microorganisms4040039. Microorganisms. 2016. PMID: 27754466 Free PMC article. Review.
-
Alternative strategies for Chlamydia treatment: Promising non-antibiotic approaches.Front Microbiol. 2022 Nov 23;13:987662. doi: 10.3389/fmicb.2022.987662. eCollection 2022. Front Microbiol. 2022. PMID: 36504792 Free PMC article. Review.
-
Advances in the Role of Antimicrobial Peptides in the Management of Sexually Transmitted Infections.J Clin Lab Anal. 2025 Jun;39(11):e70041. doi: 10.1002/jcla.70041. Epub 2025 May 14. J Clin Lab Anal. 2025. PMID: 40366090 Free PMC article. Review.
-
The cell-penetrating peptide, Pep-1, has activity against intracellular chlamydial growth but not extracellular forms of Chlamydia trachomatis.J Antimicrob Chemother. 2009 Jan;63(1):115-23. doi: 10.1093/jac/dkn436. Epub 2008 Oct 27. J Antimicrob Chemother. 2009. PMID: 18957395 Free PMC article.
References
-
- Bechinger, B. 1997. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197–211. - PubMed
-
- Boisvert, J. F., L. A. Koutsky, R. J. Suchland, and W. E. Stamm. 1999. Clinical features of Chlamydia trachomatis rectal infection by serovar among homosexually active men. Sex. Transm. Dis. 26:392–398. - PubMed
-
- Boman, H. G. 1998. Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand J. Immunol 48:15–25. - PubMed
-
- Centers for Disease Control and Prevention. 1999. Summary of notifiable diseases, United States, 1998. Morb. Mortal. Wkly. Rep. 47:11–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

