Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin
- PMID: 11751573
- PMCID: PMC1083921
- DOI: 10.1093/embo-reports/kvf002
Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin
Abstract
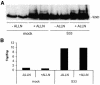
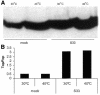
beta-catenin mediates Wnt signaling by acting as the essential co-activator for TCF transcription factors. Wnt signaling increases the half-life and therefore the absolute level of beta-catenin in responding cells. The current model states that these changes in beta-catenin stability set the threshold for Wnt signaling. However, we find that pharmacological inhibition of proteasome activity by ALLN leads to accumulation of cytosolic beta-catenin but not to increased TCF-mediated transcription. In addition, in temperature-sensitive ubiquitylation mutant CHO cells inhibition of ubiquitylation increases beta-catenin levels, but does not induce transcriptional activation of TCF reporter genes. Using an antibody specific for beta-catenin dephosphorylated at residues Ser37 and Thr41, we show that Wnt signals specifically increase the levels of dephosphorylated beta-catenin, whereas ALLN does not. We conclude that changes in the phosphorylation status of the N-terminus of beta-catenin that occur upon Wnt signaling independently affect the signaling properties and half-life of beta-catenin. Hence, Wnt signals are transduced via N-terminally dephosphorylated beta-catenin.
Figures
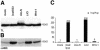


References
-
- Behrens J., von Kries, J.P., Kuhl, M., Bruhn, L., Wedlich, D., Grosschedl, R. and Birchmeier, W. (1996) Functional interaction of b-catenin with the transcription factor LEF-1. Nature, 382, 638–642. - PubMed
-
- Behrens J., Jerchow, B.A., Wurtele, M., Grimm, J., Asbrand, C., Wirtz, R., Kuhl, M., Wedlich, D. and Birchmeier, W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science, 280, 596–599. - PubMed
-
- Brunner E., Peter, O., Schweizer, L. and Basler, K. (1997) Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature, 385, 829–833. - PubMed
-
- Devereux T.R., Anna, C.H., Foley, J.F., White, C.M., Sills, R.C. and Barrett, J.C. (1999) Mutation of β-catenin is an early event in chemically induced mouse hepatocellular carcinogenesis. Oncogene, 18, 4726–4733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

