Methylation at arginine 17 of histone H3 is linked to gene activation
- PMID: 11751582
- PMCID: PMC1083932
- DOI: 10.1093/embo-reports/kvf013
Methylation at arginine 17 of histone H3 is linked to gene activation
Abstract
The nuclear hormone receptor co-activator CARM1 has the potential to methylate histone H3 at arginine residues in vitro. The methyltransferase activity of CARM1 is necessary for its co-activator functions in transient transfection assays. However, the role of this methyltransferase in vivo is unclear, given that methylation of arginines is not easily detectable on histones. We have raised an antibody that specifically recognizes methylated arginine 17 (R17) of histone H3, the major site of methylation by CARM1. Using this antibody we show that methylated R17 exists in vivo. Chromatin immunoprecipitation analysis shows that R17 methylation on histone H3 is dramatically upregulated when the estrogen receptor-regulated pS2 gene is activated. Coincident with the appearance of methylated R17, CARM1 is found associated with the histones on the pS2 gene. Together these results demonstrate that CARM1 is recruited to an active promoter and that CARM1-mediated R17 methylation on histone H3 takes place in vivo during this active state.
Figures
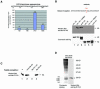
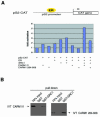

References
-
- Aletta J.M., Cimato, T.R. and Ettinger, M.J. (1998) Protein methylation: a signal event in post-translational modification. Trends Biochem. Sci., 23, 89–91. - PubMed
-
- Bannister A.J., Zegerman, P., Partridge, J.F., Miska, E.A., Thomas, J.O., Allshire, R.C. and Kouzarides, T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature, 410, 120–124. - PubMed
-
- Berger S.L. (2001) An embarrassment of niches: the many covalent modifications of histones in transcriptional regulation. Oncogene, 20, 3007–3013. - PubMed
-
- Chen D., Ma, H., Hong, H., Koh, S.S., Huang, S.M., Schurter, B.T., Aswad, D.W. and Stallcup, M.R. (1999) Regulation of transcription by a protein methyltransferase. Science, 284, 2174–2177. - PubMed
-
- Dedon P.C., Soults, J.A., Allis, C.D. and Gorovsky, M.A. (1991) A simplified formaldehyde fixation and immunoprecipitation technique for studying protein–DNA interactions. Anal. Biochem., 197, 83–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

