Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly
- PMID: 11752136
- PMCID: PMC136847
- DOI: 10.1128/jvi.76.2.467-472.2002
Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly
Abstract
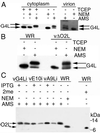
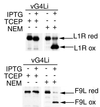
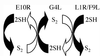
Our previous studies provided evidence that E10R, a vaccinia virus protein belonging to the ERV1/ALR family, has a redox function and is required for virion assembly. Repression of E10R prevented the formation of intramolecular disulfide bonds of the G4L glutaredoxin, the L1R membrane protein, and the structurally related F9L protein. Here, we demonstrate an oxidation pathway (E10R(SS) --> G4L(SS) --> L1R(SS), F9L(SS)) in which G4L occupies an intermediate position. By reacting free thiols with 4-acetamido-4'-malemideylstilbene-2,2'-disulfonic acid, alkylated and nonalkylated disulfide-bonded forms of G4L could be resolved from each other by polyacrylamide gel electrophoresis. The cysteines of intracellular G4L were in both disulfide and reduced forms, whereas those of E10R, L1R, and F9L and virion-associated G4L were mostly disulfide bonded. Repression of G4L expression prevented the formation of disulfide bonds in both L1R and F9L but not E10R. Both cysteines of G4L were required for L1R and F9L disulfide bond formation or for trans-complementation of virus infectivity when G4L expression was repressed. No role in the E10R-G4L redox pathway was found for O2L, a nonessential glutaredoxin encoded by vaccinia virus. We suggest that cytoplasmic G4L is a redox shuttle between membrane-associated E10R and L1R or F9L.
Figures



Similar articles
-
A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation.Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12068-73. doi: 10.1073/pnas.210397997. Proc Natl Acad Sci U S A. 2000. PMID: 11035794 Free PMC article.
-
Complete pathway for protein disulfide bond formation encoded by poxviruses.Proc Natl Acad Sci U S A. 2002 May 14;99(10):6667-72. doi: 10.1073/pnas.062163799. Epub 2002 Apr 30. Proc Natl Acad Sci U S A. 2002. PMID: 11983854 Free PMC article.
-
Vaccinia virus G4L gene encodes a second glutaredoxin.Virology. 1996 Dec 15;226(2):408-11. doi: 10.1006/viro.1996.0669. Virology. 1996. PMID: 8955061
-
A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis.J Virol. 2000 Oct;74(19):9175-83. doi: 10.1128/jvi.74.19.9175-9183.2000. J Virol. 2000. PMID: 10982364 Free PMC article.
-
From structure to redox: The diverse functional roles of disulfides and implications in disease.Proteomics. 2017 Mar;17(6):10.1002/pmic.201600391. doi: 10.1002/pmic.201600391. Proteomics. 2017. PMID: 28044432 Free PMC article. Review.
Cited by
-
Salmon Gill Poxvirus, the Deepest Representative of the Chordopoxvirinae.J Virol. 2015 Sep;89(18):9348-67. doi: 10.1128/JVI.01174-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136578 Free PMC article.
-
Disulfide bonding among micro 1 trimers in mammalian reovirus outer capsid: a late and reversible step in virion morphogenesis.J Virol. 2003 May;77(9):5389-400. doi: 10.1128/jvi.77.9.5389-5400.2003. J Virol. 2003. PMID: 12692241 Free PMC article.
-
Per Os Infectivity Factor 5 Identified as a Substrate of P33 in the Baculoviral Disulfide Bond Formation Pathway.J Virol. 2020 Jul 16;94(15):e00615-20. doi: 10.1128/JVI.00615-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32434885 Free PMC article.
-
Vaccinia virus A21 virion membrane protein is required for cell entry and fusion.J Virol. 2005 Aug;79(15):9458-69. doi: 10.1128/JVI.79.15.9458-9469.2005. J Virol. 2005. PMID: 16014909 Free PMC article.
-
Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway.J Virol. 2004 Mar;78(5):2348-56. doi: 10.1128/jvi.78.5.2348-2356.2004. J Virol. 2004. PMID: 14963131 Free PMC article.
References
-
- Bader, M., W. Muse, D. P. Ballou, C. Gassner, and J. C. Bardwell. 1999. Oxidative protein folding is driven by the electron transport system. Cell 98:217–227. - PubMed
-
- Earl, P. L., Cooper, N., and B. Moss. 1991. Preparation of cell cultures and vaccinia virus stocks, p.16.16.1–16.16.7. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates and Wiley International Science, New York, N.Y.
-
- Earl, P. L., N. Cooper, and B. Moss. 1991. Generation of recombinant vaccinia viruses, p.16.17.1–16.17.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates and Wiley International Science, New York, N.Y.
-
- Frand, A. R., J. W. Cuozzo, and C. A. Kaiser. 2000. Pathways for protein disulphide bond formation. Trends Cell Biol. 10:203–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

