The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype
- PMID: 11752149
- PMCID: PMC136822
- DOI: 10.1128/jvi.76.2.582-590.2002
The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype
Abstract
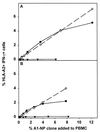
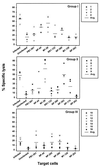
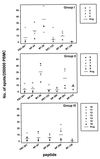
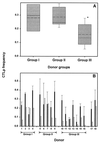
The repertoire of human cytotoxic T-lymphocytes (CTL) in response to influenza A viruses has been shown to be directed towards multiple epitopes, with a dominant response to the HLA-A2-restricted M1(58-66) epitope. These studies, however, were performed with peripheral blood mononuclear cells (PBMC) of individuals selected randomly with respect to HLA phenotype or selected for the expression of one HLA allele without considering an influence of other HLA molecules. In addition, little information is available on the influence of HLA makeup on the overall CTL response against influenza viruses. Here, the influenza A virus-specific CTL response was investigated in groups of HLA-A and -B identical individuals. Between groups the individuals shared two or three of the four HLA-A and -B alleles. After in vitro stimulation of PBMC with influenza virus, the highest CTL activity was found in HLA-A2(+) donors. A similar pattern was observed for the precursor frequency of virus-specific CTL (CTLp) ex vivo, with a higher CTLp frequency in HLA-A2-positive donors than in HLA-A2-negative donors, which were unable to recognize the immunodominant M1(58-66) epitope. In addition, CTL activity and frequency of CTLp for the individual influenza virus epitopes were determined. The frequency of CTLp specific for the HLA-B8-restricted epitope NP(380-388) was threefold lower in HLA-B27-positive donors than in HLA-B27-negative donors. In addition, the frequency of CTLp specific for the HLA-A1-restricted epitope NP(44-52) was threefold higher in HLA-A1-, -A2-, -B8-, and -B35-positive donors than in other donors, which was confirmed by measuring the CTL activity in vitro. These findings indicate that the epitope specificity of the CTL response is related to the phenotype of the other HLA molecules. Furthermore, the magnitude of the influenza virus-specific CTL response seems dependent on the HLA-A and -B phenotypes.
Figures


References
-
- Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8(+) T cell homeostasis by perforin and interferon-gamma. Science 290:1354–1358. - PubMed
-
- Bednarek, M. A., S. Y. Sauma, M. C. Gammon, G. Porter, S. Tamhankar, A. R. Williamson, and H. J. Zweerink. 1991. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J. Immunol. 147:4047–4053. - PubMed
-
- Belz, G. T., P. G. Stevenson, and P. C. Doherty. 2000. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165:2404–2409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

