Regions of the herpes simplex virus scaffolding protein that are important for intermolecular self-interaction
- PMID: 11752158
- PMCID: PMC136825
- DOI: 10.1128/jvi.76.2.673-687.2002
Regions of the herpes simplex virus scaffolding protein that are important for intermolecular self-interaction
Abstract
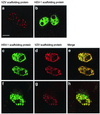
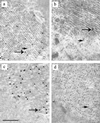
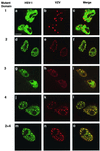
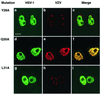
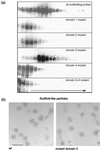
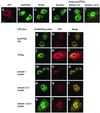
The herpes simplex virus type 1 (HSV-1) scaffolding protein encoded by gene UL26.5 promotes the formation of the icosahedral capsid shell through its association with the major capsid protein VP5 and through intermolecular interactions with itself. Inside the capsid shell, the UL26.5 product together with the maturational protease, a minor protein, form a spherical structure which is broken down and released from the capsid during packaging of the viral genome. Selected residues from four internal regions of the HSV-1 scaffolding protein that have significant conservation of amino acids within the scaffolding proteins of alphaherpesviruses were mutated, and the properties of the proteins were examined. Only the HSV-1 scaffolding protein with mutations in the conserved N-terminal domain showed reduced interaction with the varicella-zoster virus homologue in a cell-based immunofluorescence assay, providing the first evidence that this domain in the HSV-1 protein is likely to be involved in intermolecular self-interaction. Scaffolding protein with mutations in this domain or in either of two other domains failed to assemble into scaffold-like particles but retained the ability to self-interact, although the aggregates were significant smaller than most of the aggregates formed by the wild-type protein. These results suggest that there are multiple domains involved in the intermolecular self-association of the HSV-1 scaffolding protein that can act independently of one another. This conclusion was supported by the observation that none of the mutant proteins with lesions in an individual domain, including a protein with mutations in a central region previously implicated in self-interaction (A. Pelletier, F. Dô, J. J. Brisebois, L. Lagacé, and M. G. Cordingley, J. Virol. 71:5197-5208, 1997), interfered with capsid assembly in a baculovirus expression system. A protein mutated in the central region and another conserved domain, both of which had been predicted to form coiled coils, was impaired for capsid formation but still retained the capacity to interact with VP5.
Figures




Similar articles
-
Self-association of herpes simplex virus type 1 ICP35 is via coiled-coil interactions and promotes stable interaction with the major capsid protein.J Virol. 1997 Jul;71(7):5197-208. doi: 10.1128/JVI.71.7.5197-5208.1997. J Virol. 1997. PMID: 9188587 Free PMC article.
-
The 25 amino acid residues at the carboxy terminus of the herpes simplex virus type 1 UL26.5 protein are required for the formation of the capsid shell around the scaffold.J Gen Virol. 1995 Jul;76 ( Pt 7):1611-21. doi: 10.1099/0022-1317-76-7-1611. J Gen Virol. 1995. PMID: 9049368
-
Efficient herpes simplex virus type 1 (HSV-1) capsid formation directed by the varicella-zoster virus scaffolding protein requires the carboxy-terminal sequences from the HSV-1 homologue.J Gen Virol. 1997 Jul;78 ( Pt 7):1633-46. doi: 10.1099/0022-1317-78-7-1633. J Gen Virol. 1997. PMID: 9225040
-
Herpesvirus capsid assembly: insights from structural analysis.Curr Opin Virol. 2011 Aug;1(2):142-9. doi: 10.1016/j.coviro.2011.06.003. Curr Opin Virol. 2011. PMID: 21927635 Free PMC article. Review.
-
The herpes simplex virus type 1 particle: structure and molecular functions. Review article.APMIS. 1994 May;102(5):321-46. doi: 10.1111/j.1699-0463.1994.tb04882.x. APMIS. 1994. PMID: 8024735 Review.
Cited by
-
The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome.J Virol. 2008 Jul;82(13):6654-66. doi: 10.1128/JVI.00257-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448531 Free PMC article.
-
The amino-conserved domain of human cytomegalovirus UL80a proteins is required for key interactions during early stages of capsid formation and virus production.J Virol. 2007 Jan;81(2):620-8. doi: 10.1128/JVI.01903-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079329 Free PMC article.
-
A domain in the herpes simplex virus 1 triplex protein VP23 is essential for closure of capsid shells into icosahedral structures.J Virol. 2011 Dec;85(23):12698-707. doi: 10.1128/JVI.05791-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957296 Free PMC article.
-
Release of the herpes simplex virus 1 protease by self cleavage is required for proper conformation of the portal vertex.Virology. 2012 Jul 20;429(1):63-73. doi: 10.1016/j.virol.2012.03.009. Epub 2012 Apr 28. Virology. 2012. PMID: 22543049 Free PMC article.
-
pH reduction as a trigger for dissociation of herpes simplex virus type 1 scaffolds.J Virol. 2002 Aug;76(15):7407-17. doi: 10.1128/jvi.76.15.7407-7417.2002. J Virol. 2002. PMID: 12097553 Free PMC article.
References
-
- Addison, C., F. J. Rixon, J. W. Palfreyman, M. Ohara, and V. G. Preston. 1984. Characterization of a herpes simplex virus type-1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology 138:246–259. - PubMed
-
- Casjens, S., and R. Hendrix. 1988. Control mechanisms in dsDNA bacteriophage assembly, p.15–91. In R. Calendar (ed.), The bacteriophages, vol. 1. Plenum Press, New York, N.Y.
-
- Chou, P. Y., and G. D. Fasman. 1978. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. 47:45–147. - PubMed
-
- Desai, P., and S. Person. 1996. Molecular interactions between the HSV-1 capsid proteins as measured by the yeast two-hybrid system. Virology 220:516–521. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

