Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity
- PMID: 11752169
- PMCID: PMC136844
- DOI: 10.1128/jvi.76.2.791-801.2002
Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity
Abstract
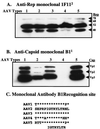
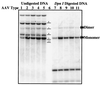
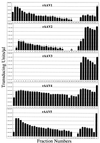
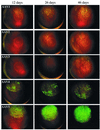
The serotypes of adeno-associated virus (AAV) have the potential to become important resources for clinical gene therapy. In an effort to compare the role of serotype-specific virion shells on vector transduction, we cloned each of the serotype capsid coding domains into a common vector backbone containing AAV type 2 replication genes. This strategy allowed the packaging of AAV2 inverted terminal repeat vectors into each serotype-specific virions. Each of these helper plasmids (pXR1 through pXR5) efficiently replicated the transgene DNA and expressed helper proteins at nearly equivalent levels. In this study, we observed a correlation between the amount of transgene replication and packaging efficiency. The physical titer of these hybrid vectors ranged between 1.3 x 10(11) and 9.8 x 10(12)/ml (types 1 and 2, respectively). Of the five serotype vectors, only types 2 and 3 were efficiently purified by heparin-Sepharose column chromatography, illustrating the high degree of similarity between these virions. We analyzed vector transduction in reference and mutant Chinese hamster ovary cells deficient in heparan sulfate proteoglycan and saw a correlation between transduction and heparan sulfate binding data. In this analysis, types 1 and 5 were most consistent in transduction efficiency across all cell lines tested. In vivo each serotype was ranked after comparison of transgene levels by using different routes of injection and strains of rodents. Overall, in this analysis, type 1 was superior for efficient transduction of liver and muscle, followed in order by types 5, 3, 2, and 4. Surprisingly, this order changed when vector was introduced into rat retina. Types 5 and 4 were most efficient, followed by type 1. These data established a hierarchy for efficient serotype-specific vector transduction depending on the target tissue. These data also strongly support the need for extending these analyses to additional animal models and human tissue. The development of these helper plasmids should facilitate direct comparisons of serotypes, as well as begin the standardization of production for further clinical development.
Figures

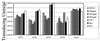
References
-
- Auricchio, A., M. Hildinger, E. O’Conner, G. Gao, and J. Wilson. 2001. Isolation of highly infectious and pure adeno-associalted virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 12:71–76. - PubMed
-
- Bartlett, J. S., J. Kleinschmidt, R. S. Boucher, and R. J. Samulski. 1999. Targeted adeno-associated virus vector transduction of non-permissive cells mediated by bispecific F(ab′γ)2 antibody. Nat. Biotechnol. 17:181–186. - PubMed
-
- Carter, B. J., and C. A. Laughlin. 1984. Adeno-associated virus defectiveness and the nature of the adenovirus helper function, p.67–152. In K. I. Berns (ed.), The parvoviruses. Plenum Press, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

