Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection
- PMID: 11752172
- PMCID: PMC136836
- DOI: 10.1128/jvi.76.2.829-840.2002
Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection
Abstract
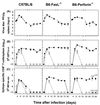
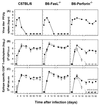
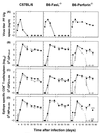
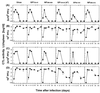
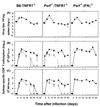
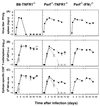
Viral persistence following infection with invasive strains of lymphocytic choriomeningitis virus (LCMV) can be achieved by selective down-regulation of virus-specific T lymphocytes. High viral burden in the onset of infection drives responding cells into functional unresponsiveness (anergy) that can be followed by their physical elimination. In this report, we studied down-regulation of the virus-specific CD8(+)-T-cell response during persistent infection of adult mice with LCMV, with emphasis on the role of perforin-, Fas/FasL-, or tumor necrosis factor receptor 1 (TNFR1)-mediated cytolysis in regulating T-cell homeostasis. The results reveal that the absence of perforin, Fas-ligand, or TNFR1 has no significant effect on the kinetics of proliferation and functional inactivation of virus-specific CD8(+) T cells in the onset of chronic LCMV infection. However, these molecules play a critical role in the homeostatic regulation of T cells, influencing the longevity of the virus-specific CD8(+)-T-cell population once it has become anergic. Thus, CD8(+) T cells specific to the dominant LCMV NP(396-404) epitope persist in an anergic state for at least 70 days in perforin-, FasL-, or TNFR1-deficient mice, but they were eliminated by day 30 in C57BL/6 controls. These effects were additive as shown by a deficit of apoptotic death of NP(396-404) peptide-specific CD8(+) T cells in mice lacking both perforin and TNFR1. This suggests a role for perforin-, FasL-, and TNFR1-mediated pathways in down-regulation of the antiviral T cell response during persistent viral infection by determining the fate of antigen-specific T cells. Moreover, virus-specific anergic CD8(+) T cells in persistently infected C57BL/6 mice contain higher levels of Bcl-2 and Bcl-XL than functionally intact T cells generated during acute LCMV infection. In the case of proapoptotic factors, Bax expression did not differ between T-cell populations and Bad was below the limit of detection in all samples. As expression of the Bcl-2 family members controls susceptibility to apoptosis, this finding may provide a molecular basis for the survival of anergic cells under conditions of prolonged antigen stimulation.
Figures


References
-
- Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Persistence of viruses, p.219–249. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven, Philadelphia, Pa.
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. A. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleen of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540. - PMC - PubMed
-
- Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

