Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro
- PMID: 11752173
- PMCID: PMC136846
- DOI: 10.1128/jvi.76.2.841-850.2002
Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro
Abstract
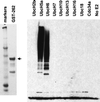
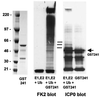
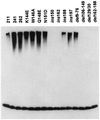
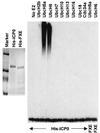
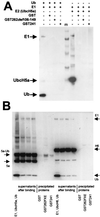
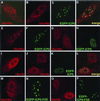
Proteasome-dependent degradation of ubiquitinated proteins plays a key role in many important cellular processes. Ubiquitination requires the E1 ubiquitin activating enzyme, an E2 ubiquitin conjugating enzyme, and frequently a substrate-specific ubiquitin protein ligase (E3). One class of E3 ubiquitin ligases has been shown to contain a common zinc-binding RING finger motif. We have previously shown that herpes simplex virus type 1 ICP0, itself a RING finger protein, induces the proteasome-dependent degradation of several cellular proteins and induces the accumulation of colocalizing conjugated ubiquitin in vivo. We now report that both full-length ICP0 and its isolated RING finger domain induce the accumulation of polyubiquitin chains in vitro in the presence of E1 and the E2 enzymes UbcH5a and UbcH6. Mutations within the RING finger region that abolish the in vitro ubiquitination activity also cause severe reductions in ICP0 activity in other assays. We conclude that ICP0 has the potential to act as an E3 ubiquitin ligase during viral infection and to target specific cellular proteins for destruction by the 26S proteasome.
Figures
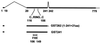
References
-
- Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935–941. - PubMed
-
- Everett, R. D. 1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 202:87–96. - PubMed
-
- Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

