Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection
- PMID: 11752176
- PMCID: PMC136839
- DOI: 10.1128/jvi.76.2.875-884.2002
Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection
Abstract
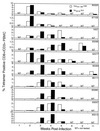
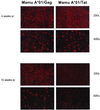
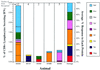
Cytotoxic T-lymphocyte (CTL) responses are thought to control human immunodeficiency virus replication during the acute phase of infection. Understanding the CD8(+) T-cell immune responses early after infection may, therefore, be important to vaccine design. Analyzing these responses in humans is difficult since few patients are diagnosed during early infection. Additionally, patients are infected by a variety of viral subtypes, making it hard to design reagents to measure their acute-phase immune responses. Given the complexities in evaluating acute-phase CD8(+) responses in humans, we analyzed these important immune responses in rhesus macaques expressing a common rhesus macaque major histocompatibility complex class I molecule (Mamu-A*01) for which we had developed a variety of immunological assays. We infected eight Mamu-A*01-positive macaques and five Mamu-A*01-negative macaques with the molecularly cloned virus SIV(mac)239 and determined all of the simian immunodeficiency virus-specific CD8(+) T-cell responses against overlapping peptides spanning the entire virus. We also monitored the evolution of particular CD8(+) T-cell responses by tetramer staining of peripheral lymphocytes as well as lymph node cells in situ. In this first analysis of the entire CD8(+) immune response to autologous virus we show that between 2 and 12 responses are detected during the acute phase in each animal. CTL against the early proteins (Tat, Rev, and Nef) and against regulatory proteins Vif and Vpr dominated the acute phase. Interestingly, CD8(+) responses against Mamu-A*01-restricted epitopes Tat(28-35)SL8 and Gag(181-189)CM9 were immunodominant in the acute phase. After the acute phase, however, this pattern of reactivity changed, and the Mamu-A*01-restricted response against the Gag(181-189)CM9 epitope became dominant. In most of the Mamu-A*01-positive macaques tested, CTL responses against epitopes bound by Mamu-A*01 dominated the CD8(+) cellular immune response.
Figures
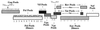



References
-
- Addo, M. M., M. Altfeld, E. S. Rosenberg, R. L. Eldridge, M. N. Philips, K. Habeeb, A. Khatri, C. Brander, G. K. Robbins, G. P. Mazzara, P. J. Goulder, and B. D. Walker. 2001. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals Proc. Natl. Acad. Sci. USA 98:1781–1786. - PMC - PubMed
-
- Allen, T. M., D. H. O’Connor, P. Jing, J. L. Dzuris, B. R. Mothe, E. Dunphy, M. E. Liebl, T. U. Vogel, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific CTL select for SIV escape variants during resolution of primary viremia. Nature 407:386–390. - PubMed
-
- Altfeld, M. A., A. Trocha, R. L. Eldridge, E. S. Rosenberg, M. N. Phillips, M. M. Addo, R. P. Sekaly, S. A. Kalams, S. A. Burchett, K. McIntosh, B. D. Walker, and P. J. Goulder. 2000. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74:8541–8549. - PMC - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O’Neil, S. J. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69–74. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

