Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease
- PMID: 11752178
- PMCID: PMC136833
- DOI: 10.1128/jvi.76.2.895-904.2002
Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease
Abstract
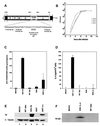
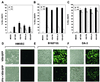
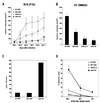
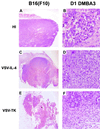
We report here the generation of recombinant vesicular stomatitis virus (VSV) able to produce the suicide gene product thymidine kinase (TK) or cytokine interleukin 4 (IL-4). In vitro cells infected with the engineered viruses expressed remarkably high levels of biologically active TK or IL-4 and showed no defects in replication compared to the wild-type virus. Recombinant viruses retained their ability to induce potent apoptosis in a variety of cancer cells, while normal cells were evidently more resistant to infection and were completely protected by interferon. Significantly, following direct intratumoral inoculation, VSV expressing either TK or IL-4 exhibited considerably more oncolytic activity against syngeneic breast or melanoma tumors in murine models than did the wild-type virus or control recombinant viruses expressing green fluorescent protein (GFP). Complete regression of a number of tumors was achieved, and increased granulocyte-infiltrating activity with concomitant, antitumor cytotoxic T-cell responses was observed. Aside from discovering greater oncolytic activity following direct intratumoral inoculation, however, we also established that VSV expressing IL-4 or TK, but not GFP, was able to exert enhanced antitumor activity against metastatic disease. Following intravenous administration of the recombinant viruses, immunocompetent BALB/c mice inoculated with mammary adenocarcinoma exhibited prolonged survival against lethal lung metastasis. Our data demonstrate the validity of developing novel types of engineered VSV for recombinant protein production and as a gene therapy vector for the treatment of malignant and other disease.
Figures

References
-
- Asnagli, H., and K. M. Murphy. 2001. Stability and commitment in T helper cell development. Curr. Opin. Immunol. 13:242–247. - PubMed
-
- Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135–138. - PubMed
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129–141. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

