Optical imaging of Renilla luciferase reporter gene expression in living mice
- PMID: 11752410
- PMCID: PMC117568
- DOI: 10.1073/pnas.012611099
Optical imaging of Renilla luciferase reporter gene expression in living mice
Abstract
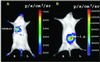
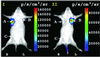
Imaging reporter gene expression in living subjects is a rapidly evolving area of molecular imaging research. Studies have validated the use of reporter genes with positron emission tomography (PET), single photon emission computed tomography (SPECT), MRI, fluorescence with wild-type and mutants of green fluorescent protein, as well as bioluminescence using Firefly luciferase enzyme/protein (FL). In the current study, we validate for the first time the ability to image bioluminescence from Renilla luciferase enzyme/protein (RL) by injecting the substrate coelenterazine in living mice. A highly sensitive cooled charge-coupled device camera provides images within a few minutes of photon counting. Cells, transiently expressing the Rluc were imaged while located in the peritoneum, s.c. layer, as well as in the liver and lungs of living mice tail-vein injected with coelenterazine. Furthermore, d-luciferin (a substrate for FL) does not serve as a substrate for RL, and coelenterazine does not serve as a substrate for FL either in cell culture or in living mice. We also show that both Rluc and Fluc expression can be imaged in the same living mouse and that the kinetics of light production are distinct. The approaches validated will have direct applications to various studies where two molecular events need to be tracked, including cell trafficking of two cell populations, two gene therapy vectors, and indirect monitoring of two endogenous genes through the use of two reporter genes.
Figures


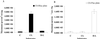


References
-
- Ray P, Bauer E, Iyer M, Barrio J R, Satyamurthy N, Phelps M E, Herschman H R, Gambhir S. Semin Nucl Med. 2001;31:321–320. - PubMed
-
- Bremer C, Weissleder R. Acad Radiol. 2001;8:15–23. - PubMed
-
- Contag C H, Spilman S D, Contag P R, Oshiro M, Eames B, Dennery P, Stevenson D K, Benaron D A. Photochem Photobiol. 1997;66:523–531. - PubMed
-
- Contag P R, Olomu I N, Stevenson D K, Contag C H. Nat Med. 1998;4:245–247. - PubMed
-
- Lipshutz G S, Gruber C A, Cao Y, Hardy J, Contag C H, Gaensler K M L. Mol Ther. 2001;3:284–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

