Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1
- PMID: 11752411
- PMCID: PMC117578
- DOI: 10.1073/pnas.221439999
Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1
Abstract
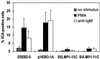
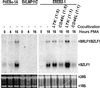
In humans, Epstein-Barr virus (EBV) establishes a persistent latent infection in peripheral resting B lymphocytes. Virus reactivation is highly restricted. Whereas in healthy humans the infection usually is benign, immunocompromised patients show an increased risk for EBV-associated malignancies, accompanied by an increase in virus replication and in the number of virus-infected cells. To search for viral and host factors regulating virus reactivation, we used conditionally EBV-immortalized B cells. We found that CD40-CD40 ligand interaction and the viral mimic of activated CD40, EBV latent membrane protein 1, suppress virus reactivation. Both inhibit anti-IgM or phorbolester-induced transcription of the viral immediate early protein BZLF1, which controls entry into the viral lytic cycle. The finding that latent membrane protein 1 and CD40 contribute to the regulation of latency may have important implications for the balance between EBV and its host in normal as well as in immunocompromised individuals.
Figures



References
-
- Rickinson A B, Kieff E. In: Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Raven; 1996. pp. 2397–2446.
-
- Falk K, Ernberg L, Sakthivel R, Davis J, Christensson B, Luka J, Okano M, Grierson H L, Klein G, Purtilo D T. Int J Cancer. 1990;46:976–984. - PubMed
-
- Kieff E. In: Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Raven; 1996. pp. 2343–2396.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

