The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP-ribose
- PMID: 11752433
- PMCID: PMC64949
- DOI: 10.1073/pnas.261448698
The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP-ribose
Abstract
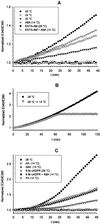
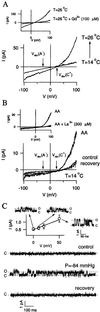
Sponges (phylum Porifera) are the phylogenetically oldest metazoan animals, their evolution dating back to 600 million years ago. Here we demonstrate that sponges express ADP-ribosyl cyclase activity, which converts NAD(+) into cyclic ADP-ribose, a potent and universal intracellular Ca(2+) mobilizer. In Axinella polypoides (Demospongiae, Axinellidae), ADP-ribosyl cyclase was activated by temperature increases by means of an abscisic acid-induced, protein kinase A-dependent mechanism. The thermosensor triggering this signaling cascade was a heat-activated cation channel. Elucidation of the complete thermosensing pathway in sponges highlights a number of features conserved in higher organisms: (i) the cation channel thermoreceptor, sensitive to heat, mechanical stress, phosphorylation, and anesthetics, shares all of the functional characteristics of the mammalian heat-activated background K(+) channel responsible for central and peripheral thermosensing; (ii) involvement of the phytohormone abscisic acid and cyclic ADP-ribose as its second messenger is reminiscent of the drought stress signaling pathway in plants. These results suggest an ancient evolutionary origin of this stress-signaling cascade in a common precursor of modern Metazoa and Metaphyta.
Figures

References
-
- Masuda W, Takenaka S, Tsuyama S, Tokunaga M, Inui H, Miyatake K. FEBS Lett. 1997;405:104–106. - PubMed
-
- Lee H C. Physiol Rev. 1997;77:1133–1164. - PubMed
-
- Lee H C. Cell Signalling. 1994;6:591–600. - PubMed
-
- Lee H C. Biol Signals. 1996;5:101–110. - PubMed
-
- Takasawa S, Nata K, Yonekura H, Okamoto H. Science. 1993;259:370–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

