PDK1 regulates growth through Akt and S6K in Drosophila
- PMID: 11752451
- PMCID: PMC64976
- DOI: 10.1073/pnas.011318098
PDK1 regulates growth through Akt and S6K in Drosophila
Abstract
The insulin/insulin-like growth factor-1 signaling pathway promotes growth in invertebrates and vertebrates by increasing the levels of phosphatidylinositol 3,4,5-triphosphate through the activation of p110 phosphatidylinositol 3-kinase. Two key effectors of this pathway are the phosphoinositide-dependent protein kinase 1 (PDK1) and Akt/PKB. Although genetic analysis in Caenorhabditis elegans has implicated Akt as the only relevant PDK1 substrate, cell culture studies have suggested that PDK1 has additional targets. Here we show that, in Drosophila, dPDK1 controls cellular and organism growth by activating dAkt and S6 kinase, dS6K. Furthermore, dPDK1 genetically interacts with dRSK but not with dPKN, encoding two substrates of PDK1 in vitro. Thus, the results suggest that dPDK1 is required for dRSK but not dPKN activation and that it regulates insulin-mediated growth through two main effector branches, dAkt and dS6K.
Figures


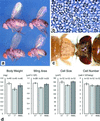

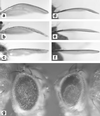
Similar articles
-
Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252.J Biol Chem. 1999 Dec 24;274(52):37400-6. doi: 10.1074/jbc.274.52.37400. J Biol Chem. 1999. PMID: 10601311
-
3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase.Curr Biol. 1997 Oct 1;7(10):776-89. doi: 10.1016/s0960-9822(06)00336-8. Curr Biol. 1997. PMID: 9368760
-
Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster.Mol Cell Biol. 2003 Dec;23(24):9117-26. doi: 10.1128/MCB.23.24.9117-9126.2003. Mol Cell Biol. 2003. PMID: 14645523 Free PMC article.
-
Intracellular signalling: PDK1--a kinase at the hub of things.Curr Biol. 1999 Feb 11;9(3):R93-6. doi: 10.1016/s0960-9822(99)80058-x. Curr Biol. 1999. PMID: 10021376 Review.
-
The PI3K-PDK1 connection: more than just a road to PKB.Biochem J. 2000 Mar 15;346 Pt 3(Pt 3):561-76. Biochem J. 2000. PMID: 10698680 Free PMC article. Review.
Cited by
-
A Quantitative Analysis of Growth and Size Regulation in Manduca sexta: The Physiological Basis of Variation in Size and Age at Metamorphosis.PLoS One. 2015 May 26;10(5):e0127988. doi: 10.1371/journal.pone.0127988. eCollection 2015. PLoS One. 2015. PMID: 26011714 Free PMC article.
-
De Novo Transcriptome Assembly and Analysis of Longevity Genes Using Subterranean Termite (Reticulitermes chinensis) Castes.Int J Mol Sci. 2022 Nov 7;23(21):13660. doi: 10.3390/ijms232113660. Int J Mol Sci. 2022. PMID: 36362447 Free PMC article.
-
The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila.Genes Dev. 2004 Dec 1;18(23):2879-92. doi: 10.1101/gad.322704. Epub 2004 Nov 15. Genes Dev. 2004. PMID: 15545626 Free PMC article.
-
Early endosomal antigen 1 (EEA1) is an obligate scaffold for angiotensin II-induced, PKC-alpha-dependent Akt activation in endosomes.J Biol Chem. 2011 Jan 28;286(4):2886-95. doi: 10.1074/jbc.M110.141499. Epub 2010 Nov 20. J Biol Chem. 2011. PMID: 21097843 Free PMC article.
-
Striking the balance between PTEN and PDK1: it all depends on the cell context.Genes Dev. 2009 Aug 1;23(15):1699-704. doi: 10.1101/gad.1832909. Genes Dev. 2009. PMID: 19651981 Free PMC article.
References
-
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. - PubMed
-
- Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, et al. Curr Biol. 1997;7:776–789. - PubMed
-
- Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Science. 1997;277:567–570. - PubMed
-
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R, Reese C B, McCormick F, Tempst P, et al. Science. 1998;279:710–714. - PubMed
-
- Belham C, Wu S, Avruch J. Curr Biol. 1999;9:R93–R96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

