Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus
- PMID: 11752458
- PMCID: PMC64994
- DOI: 10.1073/pnas.011593298
Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus
Erratum in
- Proc Natl Acad Sci U S A 2002 Feb 19;99(4):2460
Abstract
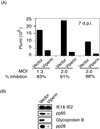
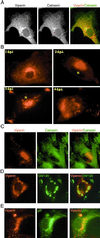
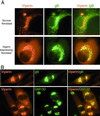
Little is known about the mechanism by which IFNs inhibit human cytomegalovirus (HCMV) replication. Indeed, infection of fibroblasts with HCMV initiates the expression of a subset of type I IFN-inducible genes whose role in the infectious process is unclear. We describe here the identification of a cytoplasmic antiviral protein that is induced by IFNs, by HCMV infection, and by the HCMV envelope protein, glycoprotein B (gB). Stable expression of the protein in fibroblasts inhibits productive HCMV infection, down-regulating several HCMV structural proteins (gB, pp28, and pp65) known to be indispensable for viral assembly and maturation. We have named the protein viperin (for virus inhibitory protein, endoplasmic reticulum-associated, interferon-inducible). HCMV infection causes the redistribution of the induced viperin from its normal endoplasmic reticulum association, first to the Golgi apparatus and then to cytoplasmic vacuoles containing gB and pp28. Expression before HCMV infection reduces viperin redistribution from the endoplasmic reticulum to the Golgi apparatus and prevents vacuolar localization, perhaps reflecting the mechanism used by HCMV to evade the antiviral function.
Figures



References
-
- Britt W J, Alford C A. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott-Raven; 1996.
-
- Ho M. Cytomegalovirus: Biology and Infection. New York: Plenum; 1991.
-
- Klatt E C, Shibata D. Arch Pathol Lab Med. 1988;112:540–544. - PubMed
-
- Rubin R H. Rev Infect Dis. 1990;12, Suppl. 7:S754–S766. - PubMed
-
- Winston D J, Ho W G, Champlin R E. Rev Infect Dis. 1990;12, Suppl. 7:S776–S792. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

