Age and stage dependency of estrogen receptor expression by lymphocyte precursors
- PMID: 11752459
- PMCID: PMC64995
- DOI: 10.1073/pnas.011513098
Age and stage dependency of estrogen receptor expression by lymphocyte precursors
Abstract
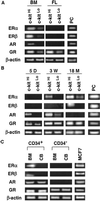
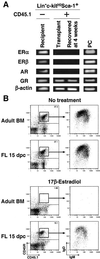
Sex steroids negatively regulate B lymphopoiesis in adult mice. Paradoxically, lymphocytes arise during fetal life, when estrogen levels are high and maternal lymphopoiesis is suppressed. Here we demonstrate that embryonic B lymphopoiesis was unaffected by estrogen, but sensitive to glucocorticoids. Both fetal and adult precursors contained glucocorticoid receptor transcripts, but only adult precursors expressed estrogen receptor alpha and beta together with the androgen receptor. Fetal hematopoietic cells did not efficiently acquire functional estrogen receptors after transplantation to irradiated adult mice. Sex steroid receptors were also expressed in a stage- and developmental age-dependent fashion in human precursors. A developmental switch in responsiveness of hematopoietic cells to sex steroids may be essential for formation of the immune system.
Figures


Similar articles
-
The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis.J Immunol. 1998 Jul 1;161(1):27-34. J Immunol. 1998. PMID: 9647203
-
Estrogen suppresses stromal cell-dependent lymphopoiesis in culture.J Immunol. 1995 Oct 1;155(7):3409-17. J Immunol. 1995. PMID: 7561035
-
Differential expression of estrogen receptor-related receptor alpha and estrogen receptors alpha and beta in osteoblasts in vivo and in vitro.J Bone Miner Res. 2002 Aug;17(8):1392-400. doi: 10.1359/jbmr.2002.17.8.1392. J Bone Miner Res. 2002. PMID: 12162493
-
B-lymphopoiesis gains sensitivity to subsequent inhibition by estrogens during final phase of fetal development.Dev Comp Immunol. 2012 Feb;36(2):385-9. doi: 10.1016/j.dci.2011.07.009. Epub 2011 Aug 10. Dev Comp Immunol. 2012. PMID: 21854803
-
The clinical and pathogenetic significance of estrogen receptor-beta expression in chronic liver diseases and liver carcinoma.Cancer. 2003 Aug 1;98(3):529-34. doi: 10.1002/cncr.11528. Cancer. 2003. PMID: 12879470
Cited by
-
Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation.J Immunol. 2009 May 1;182(9):5846-54. doi: 10.4049/jimmunol.0801458. J Immunol. 2009. PMID: 19380833 Free PMC article.
-
Research resource: Comparative nuclear receptor atlas: basal and activated peritoneal B-1 and B-2 cells.Mol Endocrinol. 2011 Mar;25(3):529-45. doi: 10.1210/me.2010-0384. Epub 2011 Jan 27. Mol Endocrinol. 2011. PMID: 21273443 Free PMC article.
-
Perinatal glucocorticoid sensitivity in the preterm newborn: molecular mechanisms, endogenous determinants, and clinical implications.Front Endocrinol (Lausanne). 2025 Jul 16;16:1587891. doi: 10.3389/fendo.2025.1587891. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40741174 Free PMC article. Review.
-
Further differentiation of murine double-positive thymocytes is inhibited in adenosine deaminase-deficient murine fetal thymic organ culture.J Immunol. 2006 May 15;176(10):5925-33. doi: 10.4049/jimmunol.176.10.5925. J Immunol. 2006. PMID: 16670300 Free PMC article.
-
Biological sex: an understudied factor driving disease susceptibility in pigs.J Anim Sci. 2022 Jun 1;100(6):skac146. doi: 10.1093/jas/skac146. J Anim Sci. 2022. PMID: 35708590 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

